Proceedings of the 24th Meeting
Working Group on Prolamin Analysis
and Toxicity (PWG)
Edited by Mar tin Stern
University of Tübingen
Verlag Wissenschaftliche Scripten - 2011
Introduction
The 24th meeting of the Working Group on Prolamin Analysis and Toxicity (PWG) took place at the University of Ancona, Facoltà di Economia, Ancona, Italy, from September 30 to October 2, 2010. Our hosts, Professor Carlo Catassi from Università Politecnica delle Marche and AiC - Associazione Italiana Celiachia, Genova, Italy, as well as Elisa Giordano from forum service, Genova, Italy, welcomed the group, the invited speakers and participants from industry (cereal starch producers, producers of gluten-free food, manufacturers of kits for gluten analysis) as well as representatives from international and national coeliac societies who attended the meeting.
The Prolamin Group meeting aimed at continuing the analytical and clinical discussion
initiated by Codex Alimentarius concerning gluten analysis and the control of food for
special dietary use for persons intolerant to gluten. A special symposium was devoted to
gluten sensitivity beyond coeliac disease and to novel therapies.
I am grateful to all participants for their active contributions, in particular to Carlo
Catassi, AiC and forum service for their kind hospitality and efficient organisation of
the meeting. I express my gratitude towards all friends, colleagues and sponsors for
their inspiration and continued support.
January 2011, Tübingen Martin Stern
Executive Summary
The meeting focused on gluten analysis and the control of food for special dietary use
for persons intolerant to gluten. Beyond this the spectrum of gluten sensitivity was
extended and novel alternative therapies were discussed.
Analytical reports
Seven reports focused on ELISA methods for gluten analysis including the detection
of toxic gluten fragments. New test kits were described to be investigated further by
ring trials.
Clinical reports
Five clinical reports focused on oats, quinoa and on special findings in pathophysiology
and immunology of coeliac disease.
In a special symposium, gluten sensitivity was taken beyond the limits of coeliac
disease to include wheat allergy and a full spectrum of non-coeliac clinical forms of
gluten sensitivity, at gastrointestinal, skin and neurological level. Studies into enzymatic
fermentation including bacterial and barley peptidases and into microbial
transamidation of gluten indicated alternative ways of therapy which might be used as
an adjunct to the gluten-free diet in the future.
A statement from the Association of European Coeliac Societies (AOECS) was given.
The meeting led to a new understanding of the importance of gluten analysis.
I. Analytical research reports
Comparison of different protein references and ELISA kits
for the detection of gluten in foods
Theresa Schwalb, Her bert Wieser, Pe ter Koehler
German Re search for Food Chemistry, Freising, Germany
Introduction
Coeliac Disease (CD) is one of the most common food intolerances. It comes along with
serious damage of the mucosa in the small intestine and is caused by the storage proteins – termed ‘gluten’ – of wheat, rye, barley and, possibly, oats (for a summary cf. [1]).
These storage proteins can be distinguished into two groups: the alcohol-soluble
prolamins and the alcohol-insoluble glutelins. The determination of the presence of
gluten in foodstuffs is mainly done by means of an immunochemical method called
ELISA (enzyme-linked immunosorbent assay). This method is an approved Codexstandard
[2] that stipulates the use of an R5 antibody assay [3]. The R5 ELISA
determines the prolamin content of a sample, which is then converted into the gluten
content by multiplication with a factor of 2. There are two versions, the sandwich
ELISA for intact gluten proteins and the competitive ELISA for gluten peptides. A
number of other assays are also available to quantify gluten in food. They not only
include different antibodies but also diverse protein references for quantifying the
gluten content.
The aim of this study was to investigate the chemical and immunochemical characterization
of different protein references and to compare them with some of the ELISA kits
and lateral flow assays used for determining gluten in food- stuffs.
Material and methods
Different ELISA kits for the detection of gluten in food were compared. A sandwich
ELISA involving a monoclonal R5 antibody and the PWG gliadin as reference
material (kit no. 1, Table 1) was used as the control, and the signal intensity was set to
100% [3]. In addition, three other sandwich ELISA kits (kits no. 2 - 4, Table 1) were
used. Kit no. 2 used a monoclonal antibody according to Skerritt & Hill [4]. The
antibodies used in kits no. 3 and 4 were monoclonal and polyclonal, respectively;
however, no further information was available. In addition, two lateral flow assays
(dip sticks), based on the R5 antibody, were tested.
Samples were extracted and analyzed according to the manufacturers’ instructions.
For gluten analysis, only protein references from wheat are. Either gliadin, the
prolamin fraction of wheat, or ‘gluten’ consisting of gliadin and glutenin (the glutelin
fraction of wheat) were used. Both products are not only used as references for the
detection of gluten, but also for CD-specific medical analyses, e.g., the elucidation of
the pathomechanism [5].
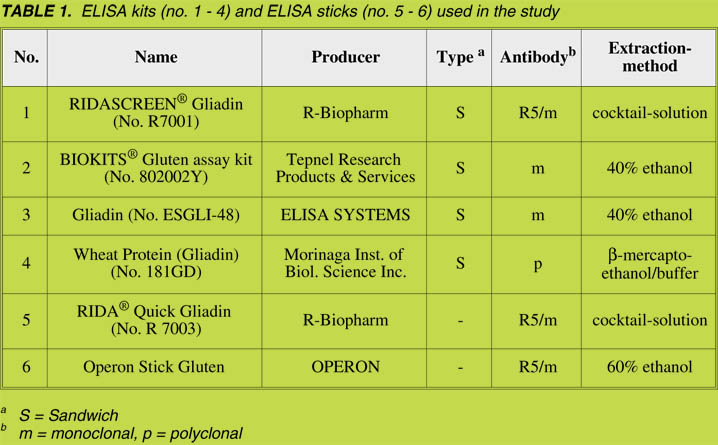
The following protein references were analyzed: ABCR ‘Gliadin’ (AB13 6288),
ABCR ‘Gluten from wheat’ (AB13 6330), SIGMA ‘Gliadin from wheat’ (G 3375),
SIGMA ‘Gluten from wheat’ (G 5004) and PWG gliadin [6]. Grains from the wheat
cultivar ‘Cubus’, the rye cultivar ‘Guttino’, the barley cultivar ‘Marthe’ and the oats
cultivar ‘Typhon’ were ground to wholemeal flours. The alcohol-soluble prolamins
and the glutelins (alcohol-soluble after reduction of the disulfide bonds) were
extracted from them and used as protein references [7]. ‘Gluten’ was washed out from
the dough of the wheat cultivar ‘Tommi’ by means of a Glutomatic and afterwards
freeze-dried [8].
The protein content (N x 5.7) of all references was determined with the Dumas method
on a nitrogen analyzer. The content of protein fractions obtained by a modified
Osborne procedure was analyzed by means of RP-HPLC [7].
Results and discussion
Reference proteins
As determined by means of the Dumas method, the crude protein content of the
protein fractions was between 61% and 95% (Table 2). This had to be taken into
account when these fractions were used for analytical purposes because different
amounts of material were necessary to ensure the same amount of protein in the
experiments.
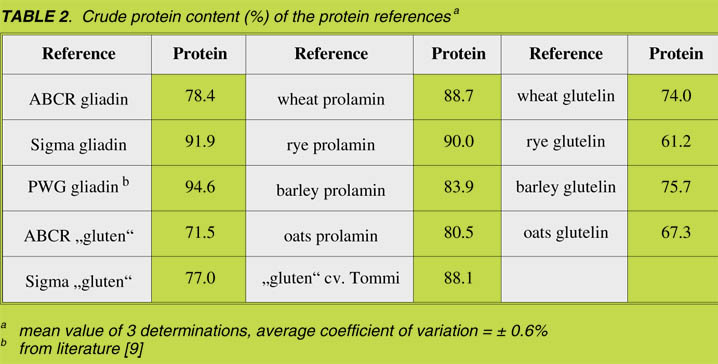
For further characterization the commercially-available proteins were analyzed for
their content of salt-soluble albumins/globulins, alcohol-soluble prolamins and
alcohol-insoluble glutelins by means of a modified Osborne fractionation with
subsequent HPLC analysis [7]. The results showed that all materials, even the samples
declared as pure gliadin, contained considerable amounts (25.4% – 63.8%) of
alcohol-insoluble glutelin [Fig. 1]. Thus, different materials (i.e., ABCR ‘Gliadin’ and SIGMA ‘Gluten’) had almost the same composition.
In summary, the protein analytical investigations showed that the protein references
available on the market featured significant differences in terms of the content of
crude protein as well as in the composition of Osborne-type protein fractions.
Therefore, it can be concluded that the calibration of ELISA kits with these references
would lead to different results in the determination of gluten.
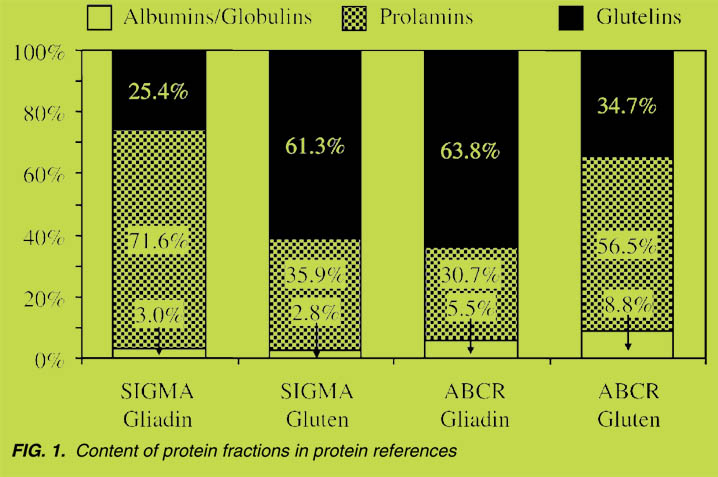
Comparison of ELISA kits
Before comparing the protein references, all ELISA kits were calibrated with PWG
gliadin. Then, equal amounts of protein from the references (cf. Table 2) were
analyzed by means of the different ELISA kits. Sample extraction was carried out
according to the manufacturers’ instructions by using the extraction agents given in
Table 1. In the following tables, all data (%) is related to the results obtained with kit
no. 1, whose signal intensity was set to 100%.
At first, the wheat, rye, barley and oats flours were analyzed (Table 3). In general,
none of the kits was able to detect oats. In addition, the analyses of wheat, rye and
barley flours showed that none among the kits no. 2 - 4 reached the values of kit no. 1.
In particular, barley flour gave low responses (4% - 23%) compared to kit no. 1. The
results of the isolated prolamin fractions were comparable to those of the flours
(Table 4). Excepting for kit no. 2, all other ELISA kits provided higher values for the
prolamin as compared to the glutelin fractions. The analysis of the commerciallyavailable
references showed considerable differences in the signal intensities ranging
from 29% - 272% (Table 5), thus reflecting the different compositions of the materials.
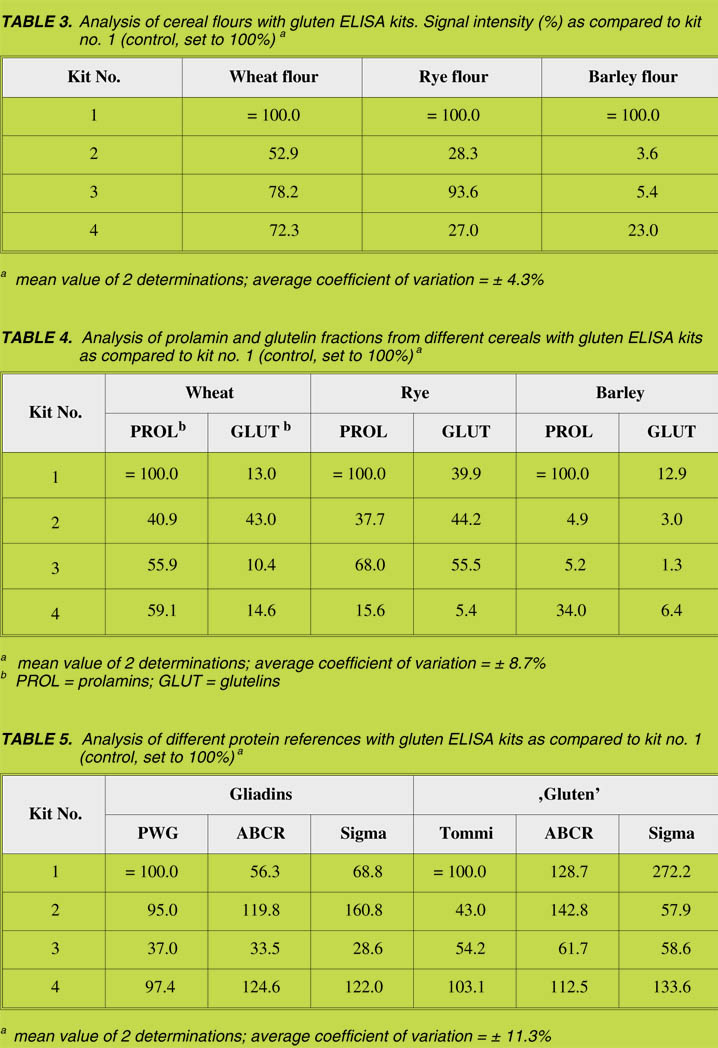
Comparison of lateral flow assays
For the easy and fast detection of gluten (prolamin) two lateral flow assays, based on
the R5 antibody (ELISA dip sticks), were compared. They were applied to different
flours and to all references listed in Table 2, according to the manufacturers’ instructions. The band on the right-hand side of the stick was the control and indicated
that the stick worked properly; whereas the band on the left indicated the presence of
gluten. As a whole, both types of sticks were comparable (results not shown) with oats
and oatmeal showing no reaction. In contrast, all the gliadin and ‘gluten’ references,
the flours from wheat, rye and barley, as well as their prolamin and glutelin fractions,
were positive (Fig. 2). The intensity of the left band correlated well with the amounts
obtained with ELISA kit no.1 (Table 4). The glutelin fractions led to considerably
weaker bands as compared to the prolamin fractions (Fig. 2).
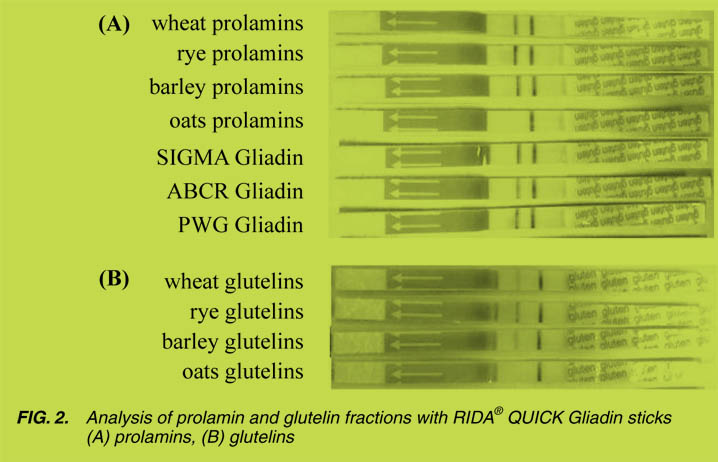
Conclusions
We concluded that the presence of gluten can be well detected by using lateral flow
assays. However, it is not yet possible to obtain the quantitative information that is
required for compliance with the threshold value of the Codex. Therefore, ELISA kits
calibrated with reliable and well-characterized reference materials should be used. We
recommend using PWG gliadin for this purpose. Commercially-available ‘gluten’ or ‘gliadin’ references show considerable differences in their composition and should,
therefore, be checked against PWG gliadin before being used.
References
1. Wieser H, Koehler P. The bio chem i cal ba sis of ce liac dis ease. Cereal Chem
2008; 85: 1-13.
2. ALINORM 08/31/26, Appendix III. Draft revised codex standard for foods for
spe cial dietary use for persons intolerant to gluten. Joint FAO/WHO Food
Stan dards Programme. Codex Alimentarius Commission, Rome: WHO 2008.
3. Valdés I, Gar cia E, Llorente M, et al. In no va tive ap proach to low-level glu ten
de ter mi na tion in foods us ing a novel sand wich en zyme-linked immunosorbent
as say pro to col. Eur J Gastroenterol Hepatol 2003; 15: 465-474.
4. Skerritt JH, Hill AS. Monoclonal an ti body sand wich en zyme immunoassays for
de ter mi na tion of glu ten in foods. J Agric Food Chem 1990; 38: 1771-1778.
5. Freitag TL, Rietdijk S, Junker Y, et al. Gliadin-primed CD4+CD45RBlowCD25-
T cells drive gluten-dependent small intestinal damage after adoptive transfer into
lymphopenic mice. Gut 2009; 58: 1597-1605.
6. Van Eckert R, Berghofer E, Ciclitira PJ, et al. To wards a new gliadin ref er ence
material – isolation and characterisation. J. Ce real Sci 2006; 43: 331-341.
7. Wieser H, An tes S, Seilmeier W. Quan ti ta tive de ter mi na tion of glu ten pro tein
types in wheat flour by re versed-phase high-per for mance liq uid chro ma tog ra phy.
Cereal Chem 1998; 75: 644-650.
8. Kieffer R, Wieser H, Henderson MH, et al. Cor re la tions of the breadmaking
per for mance of wheat flour with rhe o log i cal mea sure ments on a mi cro-scale.
J Ce real Sci 1998; 27: 53-60.
9. Appendix – PWG gliadin. In: Stern M, ed. Proceedings of the 21st Meeting of the
Work ing Group on Prolamin Analysis and Toxicity, September 28-30, 2006,
Trieste, Italy. Zwickau: Verlag Wissenschaftliche Scripten, 2007; 160.
Reactivity of different monoclonal antibodies
towards gliadins and glutenins
Renate van Eckert 1, Judy Bond 1, Pisana Raw son1, Christoph Klein 2,
Paul J. Ciclitira 3, H. Julia Ellis 3, Mar tin Stern 4, T. Wil liam Jor dan1
1 Cen tre for Biodiscovery and School of Bi o log i cal Sci ences,
Vic to ria Uni ver sity of Wellington, Wellington, New Zea land
2 Eu ro pean Com mis sion, Di rec tor ate-Gen eral, Joint Re search Cen tre
(JRC), In sti tute for Health and Con sumer Pro tec tion (IHCP), Ispra, It aly
3 King's Col lege Lon don, Di vi sion of Di a be tes and Nu tri tional Sci ences,
Rayne In sti tute, St. Thomas' Hos pi tal, Lon don, UK, Eng land
4 Uni ver sity Chil dren's Hos pi tal, Tübingen, Ger many
Abstract
The reactivity of three prominent antibodies was investigated after two-dimensional
electrophoresis (2-DE) of a gliadin material (PWG gliadin) and transfer of the proteins
via Western blot onto polyvinylidene fluoride (PVDF) membranes. Fluorescence labelling
was used for the detection of the reacting and non-reacting proteins. For this
purpose PWG gliadin was fluorescence labelled with Cy3 before 2-DE. After Western
blot the PVDF membranes were incubated with anti-gliadin mouse antibodies 401.21,
PN3 and R5 respectively. The reacting proteins were detected with a Cy5 fluorescence
labelled anti-mouse antibody. Differential scanning at two specific wavelengths for
Cy3 and Cy5 respectively showed the 2-DE pattern of the reacting and non-reacting
proteins in the same membrane. Antibodies 401.21, PN3 and R5 each detected different
protein sets of the gliadin material and thus can yield in different measurements of
gluten amounts, when used in an ELISA assay for the determination of gluten. The
findings help to explain why ELISA tests have been detecting different gluten amounts
in the past, when different test kits were used.
Introduction
Coeliac Disease is one of the most frequent food intolerances worldwide, with a
prevalence of 1 in 100 to 300 individuals [1]. The only way affected people can avoid
symptoms is by adhering strictly to a life-long, gluten-free diet. Thus a sensitive and
reliable detection method for gluten is needed. ELISA methods are taken to be the stateof-
the-art analyses for detecting gluten in gluten-free food because of their sensitivity
and specificity; however, they yield different results when different test systems are
I. Analytical research reports 31
used [2]. We investigated the reaction of three monoclonal antibodies often used for the
detection of gluten, with proteins of a gliadin material separated by 2-DE.
Materials and methods
The gliadin-material had been extracted by means of 60% (v/v) ethanol from 28 of the
most frequently-bred European wheat varieties [3]. It was named 'PWG gliadin' (this
being the abbreviated form of 'Prolamin Working Group gliadin'), because it was
initiated and produced by the Working Group on Prolamin Analysis and Toxicity.
The fluorescent labelling dye used was CyDyeTM DIGE Fluor CyTM3 (Cy3), a
minimal dye (GE-Healthcare, 25-8010-83).
The following primary antibodies were used:
1. Monoclonal an ti body (mAb) 401.21: IgG1 mouse mAb, de vel oped
against gliadin by Skerritt & Hill [4], kindly pro vided by the com pany
Vital Diag nostics Pty Ltd, Australia.
2. PN3-mAb: IgG1 mouse mAb, de vel oped against a 19-mer pep tide of
A-gliadin by Ellis et al. [5], kindly pro vided by the re search group of
Prof. Dr. Paul Ciclitira.
3. R5-mAb: IgG2b mouse mAb, developed against secalin [6], kindly
pro vided by Operon S.A., Cuarte de Huerva, Spain, via the late
Dr. Enrique Méndez.
The secondary antibody used was ECL Plex goat anti-mouse IgG, labelled with
fluorescent dye CyDyeTM DIGE Fluor CyTM5 (Cy5) (GE-Healthcare, PA 45009).
Details about the labelling of the PWG gliadin, electrophoresis, Western Blot,
antibody reaction and fluorescence scanning have been described in the published
report of van Eckert et al. [7]. A very stringent washing regime and a high
concentration of BSA in the blocking buffer were applied to avoid unspecific
reactions. The allocation of proteins to gliadin and glutenin sub-groups was made by
applying the apparent molecular weight known from our previous results and from
other published data.
The efficiency of the blot and the consistency of the protein pattern were monitored at
each stage of the procedure by means of fluorescence scanning of the Cy3-labelled
proteins.
Results
The 2-DE protein pattern was the same throughout the entire process. The spots
seemed slightly enlarged after the blot, probably due to diffusion. Some proteins in the
migration area of w gliadins and LMW glutenins were less intense after the completed
antibody reaction.
The reacting proteins on the 2-DE maps clearly showed that each antibody detected a
different set of proteins:
MAb 401.21 reacted mainly with proteins having an approximate molecular weight of
60,000 and above. It showed a reaction with HMW glutenin sub-units, presumably
LMW glutenins, w gliadins and - to a small degree - with a and g gliadins. The most
prominent reaction was observed in the HMW area.
PN3-mAb reacted mainly with proteins of an apparent molecular weight of 30,000 and
higher, which corresponds to the size a gliadins.
R5-mAb reacted strongly with a and g gliadins, especially those with a lower pI, with
the reaction with g gliadins being the strongest. It also reacted with proteins of a higher
apparent molecular weight of 50,000 and around 75,000 and higher (probably w
gliadins).
Discussion
The fluorescence technique we used was more sensitive than the Coomassie Blue stain
and allowed the scanning of protein patterns on gels and membranes at any time without
change being introduced. CyDye DIGE minimal dyes are expected to add a single dye
molecule to each protein and have a minimal effect on the charge and pI of the labelled
protein [8]. The differential scanning of the Cy3-labelled gliadin and the Cy5-labelled
antibody on the reacting proteins made it possible to measure both components in one
membrane and thus avoid gel-to-gel variation, which is a great advantage to Coomassie
Blue stained gels.
Some proteins in the w gliadin and LMWglutenin area appeared to diffuse out of the
membrane during the antibody incubation and washing regime. This is in agreement
with the results of Hurkman & Tanaka [9], who observed a reduction in colloidal
Coomassie Blue G-250 stained proteins when they were kept in water for 3 - 24 hours.
Van den Broeck et al. [10] also noticed a reduction in w gliadins, LMW glutenins and
some a gliadins when Coomassie-stained gels were destained in 10% ethanol/7.5%
acetic acid.
Our results showed that each antibody detected sets of different sub-types of gluten
proteins to a different degree. This indicates that the amount of gluten detected is
dependent on the antibody and on the reference material used. This had been assumed
in the past but could not be illustrated until now.
MAb 401.21 reacts mainly with HMW glutenins. This explains why the gliadin preparations
extracted by Wieser showed a relatively low reaction in gluten assays based
on that mAb [2]. They were obviously very pure in terms of their gliadin content and did
not contain many HMW glutenins. With our findings we can also explain why RM
8418, a gluten preparation from a Canadian spring wheat, exhibited a stronger response
than the PWG gliadin in assays based on this antibody [3]. RM 8418 is composed of
gliadins and glutenins, whereas the PWG gliadin had been extracted by means of 60%
ethanol from wheat flour so that the gliadins are strongly enriched in this material.
According to our results mAb 401.21 might be a candidate for the detection of HMW
glutenins.
PN3-mAb seems to recognise distinctly a gliadins. This relates well with the fact that
this mAb was raised against a peptide from A-gliadin, an a gliadin. It was suggested that
this mAb reacted mainly with QQQPFP [5], which is found in a, but not in g gliadins.
R5-mAb predominantly recognises the epitope with the QQPFP sequence [11]. It also
reacts with homologous repeats such as QQQFP, LQPFP and QLPFP [12]. The
QQPFP epitope occurs repeatedly in a, g and w gliadins. It has only one amino acid
less than the main reactant QQQPFP of mAb PN3, and it occurs more often in g and w
gliadins [13]. This is substantiated by our results in that mAb R5 showed a high
reaction with g gliadins. The diffusion of w gliadins from the membrane during the
incubation and washing steps of the antibody reaction might have diminished their
response.
It is not possible to completely separate gliadins and glutenins from each other
through extraction with aqueous ethanol [14]. Therefore PWG gliadin is enriched in
regard to gliadins but also contains some glutenins. It has been found that glutenins
pose the risk of coeliac toxicity as well [15]. The PWG gliadin is a valuable and
representative material in determining gliadin content. If it is characterised clearly in
regard of its glutenin content, it also can be used to determine glutenin content.
Acknowledgements
We wish to thank Dr. Herbert Wieser for providing gliadin preparations and for fruitful
discussions, Prof. Dr. Paul Ciclitira and Dr. Julia Ellis for providing the mAb PN3, Vital
Diagnostics Pty Ltd for providing mAb 401.21, Operon SA, Cuaerte de Huerva, Spain,
for providing the mAb R5 via the late Dr. Enrique Méndez, and Dr. Heinz Schimmel
(Institute for Reference Materials and Measurements of the European Commission Joint Research Centre, Geel, Belgium) for coordinating the funding (Extended characterisation
study of Gliadin from European wheat, B030333).
References
1. Wieser H, Koehler P, The bio chem i cal ba sis of coeliac dis ease.
Cereal Chem 2008; 85: 1-13.
2. Van Eckert R, Scharf M, Wald T, et al. Deter mina tion of proteins with ELISAMeth
ods: Doubt ful quan ti ta tive re sults? In: Amado R, Battaglia R, eds.
Pro ceed ings of EURO FOOD CHEM IX, 1997, Inter laken, Swit zer land.
FECS Event No. 220 (Vol.1): 263-268.
3. Van Eckert R, Berghofer E, Ciclitira P J, et al. To wards a new gliadin ref er ence
material - isolation and characterisation. J Ce real Sci 2006; 43: 331-341.
4. Skerritt JH, Hill AS. Monoclonal an ti body sand wich en zyme immunoassays for
de ter mi na tion of glu ten in foods. J Agric Food Chem 1990; 38: 1771-1778.
5. Ellis HJ, Rosen-Bronson S, O'Reilly N, et al. Mea sure ment of glu ten us ing a
monoclonal an ti body against a coeliac toxic pep tide of A-gliadin. Gut 1998;
43: 190-195.
6. Sor ell L, López JA, Valdés I, et al. An in no va tive sand wich ELISA sys tem
based on an an ti body cock tail for glu ten anal y sis. FEBS Let ters 1988; 439:
46-50.
7. Van Eckert R, Bond J, Raw son P, et al. Reactiv ity of glu ten detecting
antibod ies to a gliadin reference material. J Ce real Sci 2010; 51: 198-204.
8. Tonge R, Shaw J, Middle ton B, et al. Val i da tion and de vel op ment of
flu o res cence two-di men sional gel elec tro pho re sis proteomics tech nol ogy.
Proteomics 2001; 1: 377-396.
9. Hurkman WJ, Tanaka CK. Im proved meth ods for sep a ra tion of wheat
en do sperm pro teins and anal y sis by two-di men sional gel elec tro pho re sis.
J Cer Sci 2004; 40: 295-299.
10. Van den Broeck HC, Amer ica AHP, Smulders MJM, et al. Stain ing ef fi ciency
of spe cific pro teins de pends on the stain ing method: Wheat glu ten pro teins.
Proteomics 2008; 8: 1880-1884.
11. Valdés I, Gar cia E, Llorente M, et al. In no va tive ap proach to low-level glu ten
de ter mi na tion in foods us ing a novel sand wich en zyme-linked immunosorbent
as say pro to col. Eur J Gastroenterol Hepatol 2003; 15: 465-474.
I. Analytical research reports 35
12. Kahlenberg F, Sanchez D, Lachmann I, et al. Monoclonal an ti body R5 for
de tec tion of pu ta tively coeliac-toxic gliadin pep tides. Eur Food Res Technol
2006; 222: 78-82.
13. Osman AA, Uhlig HH, Valdés I, et al. A monoclonal an ti body that rec og nizes
a po ten tial coeliac-toxic re pet i tive pentapeptide epitope in gliadins.
Eur J Gastroenterol Hepatol 2001; 13: 1189-1193.
14. Wieser H. The pre cip i tat ing fac tor in Coeliac dis ease. Bailliére's Clinical
Gastroenterology 1995, ed. Ballière Tindall, Lon don, 9: 191-207.
15. Dewar DH, Amato M, Ellis HJ, et al. The tox ic ity of high mo lec u lar weight
glutenin sub units of wheat to pa tients with coeliac dis ease. Eur J Gastroenterol
Hepatol 2006; 18: 483-491.
A new enzyme-linked immunosorbent assay to detect
the toxic gluten fragments and proteins involved in coeliac disease
Jorge R Mujico1, Liesbeth Dekking1, Yvonne Kooy-Winkelaar1, Ron Verheijen 2, Piet van Wichen 2, Lu cia Streppel 2, Nermin Sajic 2, Jan-Wouter Drijfhout1, Frits Koning1
1 De part ment of Im mu nol ogy and Blood Trans fu sion, Leiden Uni ver sity Med i cal Cen ter, Leiden, The Neth er lands
2 EuroProxima, Arnhem, The Neth er lands
Coeliac disease (CD) is caused by inflammatory T-cell responses triggered by gluten
fragments bound to the disease-associated HLA-DQ2 or HLA-DQ8 molecules.
Gluten is a large protein family that can be subdivided into gliadins and glutenins and
both these protein families have been shown to contain multiple immunostimulatory
epitopes involved in CD. Many of the most immunogenic peptides, however, are
found in the gliadins, in particular the a-gliadins [1]. Because of its unique properties,
gluten is often used in the food industry, and gluten-free foods for CD patients must be
produced under special conditions to guarantee their safety for consumption by
patients. Levels of contamination may not exceed 20 mg/kg for foods prepared from
naturally gluten-free ingredients, and 100 mg/kg for foods rendered gluten-free.
Commercially-available enzyme-linked immunosorbent assays (ELISAs) are used to
determine the level of gluten and the most frequently used are based on the R5
monoclonal antibody (mAb), which detects gliadin sequences not involved in CD.
These kits are calibrated with a mixture of intact gluten proteins (both gliadins and
glutenins) extracted with 60% ethanol from 20 different wheat varieties, called the
Prolamin Working Group standard [2], which is, unfortunately, not a true standard as
it cannot be reproducibly generated.
To overcome these shortcomings, the Leiden University Medical Center (Leiden, The
Netherlands), in cooperation with EuroProxima (Arnhem, The Netherlands), has
developed a novel competitive ELISA, termed Gluten-Tec®. This test is based on a
mAb specific for a well-characterized T-cell stimulatory epitope of a-gliadin (a-20)
in wheat [3]. This peptide does not represent a repetitive sequence but is present only
once in a-gliadin proteins. The antibody specific for this peptide is thus well-suited
for quantification. Moreover, the mAb also detects homologue sequences present in
barley (hordein), rye (secalin) and their crossbred varieties and is thus also suitable for detection of the presence of other harmful cereals [4]. A synthetic peptide is used for
calibration, which allows an accurate and reproducible standardization. Moreover, as
the test is a competitive ELISA, not only intact but also hydrolyzed proteins can be
detected.
We have now tested the performance of the Gluten-Tec® ELISA kit by means of a
collaborative study, in accordance to the guidelines of the Association of Analytical
Communities [5]. Fifteen laboratories participated in this study, all of which were
familiar with gluten testing and/or performing of ELISAs.
The study included four different food matrices, covering a wide range of hydrolysed
and/or heat-treated food products, like two rice-based baby foods, one non-spiked and
the other spiked with 5% wheat-based baby food; maize bread, spiked with 44.2 mg/kg
of gliadin; two chocolate cake mixes, one non-spiked and the other spiked with 0.25%
gluten-containing chocolate cake mix, and one beer.
The results have confirmed that Gluten-Tec® is suitable for the measurement of T-cell
stimulatory epitopes over a wide range of concentrations and is suitable for gluten
detection in the range required to guarantee the safety of food for consumption by CD
patients. A manuscript describing the results of this study has been submitted to a peerreviewed
journal. The tests will be presented to the Codex Alimentarius as a preferred
method for gluten analysis.
Acknowledgement
This research was financed in part by the Celiac Disease Consortium, an Innovative
Cluster approved by the Dutch Genomics Initiative and partially funded by the Dutch
Government (BSIK03009).
References
1. Shan L, Qiao SW, Arentz-Hansen H, et al. Iden ti fi ca tion and anal y sis of
multivalent proteolytically resis tant pep tides from glu ten: implications for
celiac sprue. J Proteome Res 2005; 4(5): 1732-1741.
2. van Eckert R, Berghofer E, Ciclitira PJ, et al. To wards a new gliadin ref er ence
material - isolation and characterisation. J Ce real Sci 2006; 43: 331-341.
3. Vader W, Kooy,Y, van Veelen P, et al. The glu ten re sponse in chil dren with
re cent on set ce liac dis ease. A highly di verse re sponse to wards mul ti ple gliadin
and glutenin de rived pep tides. Gastroenterology 2002; 122: 1729-1737.
4. Mitea C, Kooy-Winkelaar Y, van Veelen P, et al. Fine spec i fic ity of
monoclonal an ti bod ies against ce liac dis ease-in duc ing pep tides in the
gluteome. Am J Clin Nutr 2008; 88: 1057-1066.
5. AOAC In ter na tional guide lines for col lab o ra tive study pro ce dures to val i date
characteristics of a method of analysis. J AOAC Int 1995; 78: 143-160.
Detection of toxic fragments from gluten
using a new monoclonal antibody-based test
Richard Fielder 1, Adrian Rog ers1, Elisabeth Halbmayr-Jech 2, Miguel Siglez 3
1 Romer Labs UK Ltd., The Heath Busi ness & Tech ni cal Park, Cheshire, UK
2 Romer Labs Di vi sion Hold ing GmbH, Tulln, Aus tria
3 Biomedal S. L., Sevilla, Spain
Introduction
Coeliac disease (CD) is an immune-mediated enteropathy caused by the ingestion of
gluten, a protein fraction found in certain cereals. CD occurs in genetically predisposed
persons and leads to the destruction of the microscopic finger-like projections
of the small intestine, called villi. The disease is triggered by the ingestion of peptides
from wheat, barley, rye, and, in some cases, oats. CD currently affects roughly 1% of
the world´s population, primarily adults. Immunotoxic gluten peptides, such as the
fragment called 33-mer, which are resistant to the degradation of digestive enzymes,
appear to trigger the coeliac syndrome. Homologues of this specific peptide were found
in every food grain that is toxic to CD patients, but were absent in all non-toxic food
grains [1]. A monoclonal antibody specific for a sequence occurring three times in the
immunotoxic 33-mer has been developed [2, 3]. This work summarises the results of a
new monoclonal antibody used in a lateral flow strip test that specifically recognises
the pathogenic fragment of the gliadin protein present in gluten.
Methods
The semi-quantitative immunochromatographic strip test (AgraStrip® Gluten G12,
Romer Labs UK) is based on a sandwich format. The reagents for the test and control
line are immobilised on a nitrocellulose membrane. Toxic gluten fragments in the
sample extract react with the anti-gliadin 33-mer monoclonal antibody, named G12,
which is coupled to coloured microspheres. These are pre-dried on the strip showing a
visible line when binding to immobilised anti-gliadin 33-mer monoclonal antibodies
on the test line [2, 3]. The mix of conjugate moves through the membrane to the
control line where anti-species specific antibodies, used for verifying the correct test
performance, are sprayed. Several gluten-free samples and gluten-containing samples
were analysed using the strip test. The results were confirmed by applying an ELISA,
developed ourselves, using the monoclonal G12 antibody. We also applied a
commercially-available gliadin ELISA test kit.
Results
The G12 antibody, specially developed to determine the toxic fractions present in
gluten, was used for the semi-quantitative immunochromatographic strip test. The
outstanding advantage of this new antibody in the strip test is that it enables the
detection of the actual toxic fragment of gluten due to its very high sensitivity. The
immunochromatographic test strip was compared with the ELISA methods by
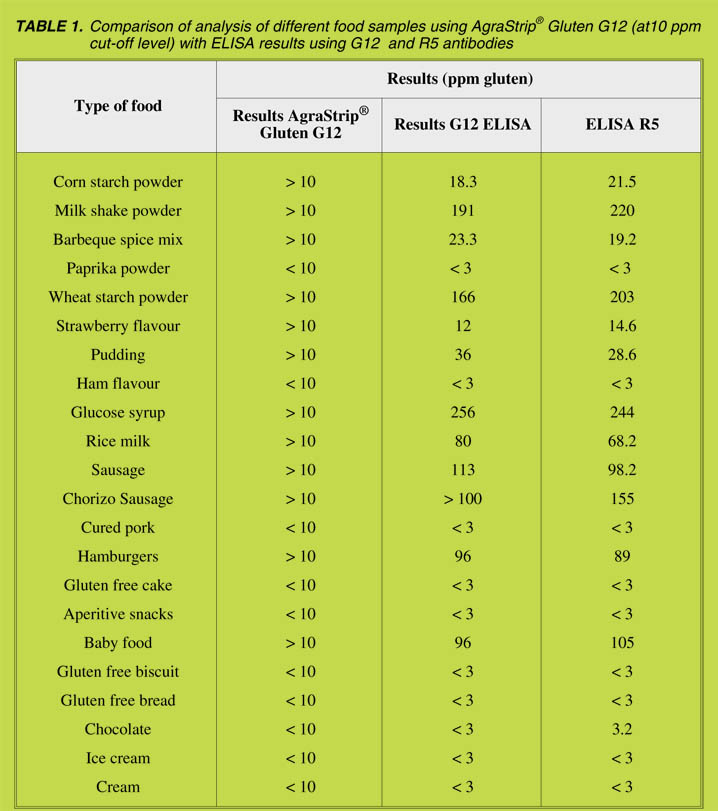
analysing a range of different food samples for their gluten content and results were
found to be similar (Table 1).
Limits of detection and cut-off
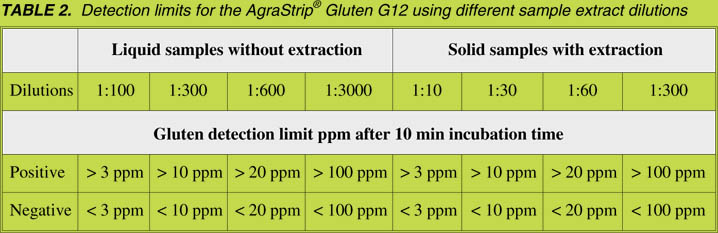
The detection limit of the AgraStrip® Gluten G12, after an incubation time of 10 min,
is 15 ng/mL gliadin. This corresponds to 3 ppm of gluten in a sample using a 1:10
extraction and 1:10 dilution of the extract. Additional cut-off levels are applicable by
using different dilutions after sample extraction (Table 2).
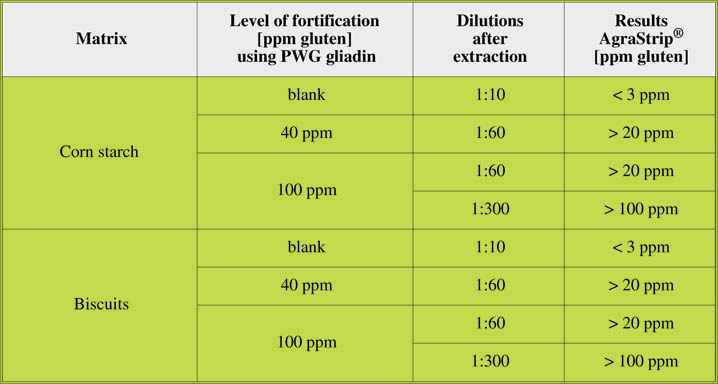
Spiked food samples
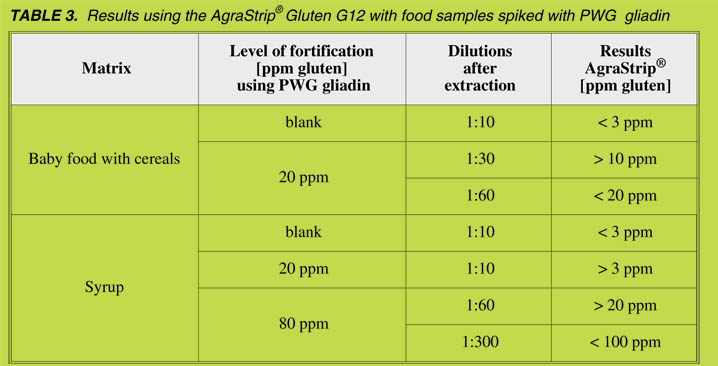
Food samples spiked with PWG gliadin were analysed using AgraStrip® Gluten G12
with different sample dilutions, resulting in different cut off levels (Table 3).
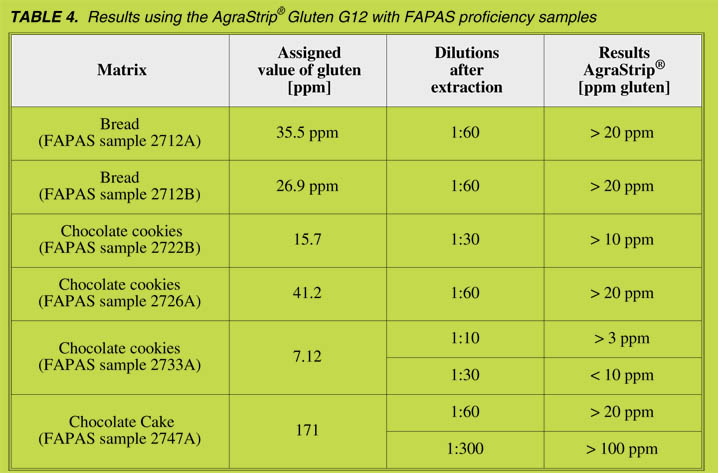
FAPAS samples
FAPAS samples with assigned values were analysed using AgraStrip® Gluten G12
with different sample dilutions, resulting in different cut-off levels (Table 4).
Conclusions
According to the current Codex Alimentarius recommendations and the Commission
Regulation (EC) No. 41/2009, food products can be labelled gluten-free if they
contain less than 20 mg/kg gluten. The lateral flow test kit (AgraStrip® Gluten G12,
Romer Labs UK), applying the monoclonal antibody named G12, can detect the toxic
fractions of gluten from wheat and other cereals such as barley and rye.
The advantage of detecting the actual toxic fragment of prolamins is that it helps
producers of gluten-free food and beverages to label the gluten content of their products
correctly. This makes the product safer for consumers suffering from CD as they have
no other option but to follow a life-long diet in which the intake of gluten is avoided.
Acknowledgements
We express our sincere thanks to all those involved in this collaborative work, in
particular Biomedal S.L., Spain; the Spanish National Research Council (CSIC);
Stanford University, USA; the University of Sevilla, Spain
References
1. Shan L, Molbergv Ø, Par rot I. et al. Struc tural ba sis for glu ten in tol er ance in
coeliac sprue. Science 2002; 297: 2275-2279.
2. Morón B, Bethune MT, Comino I. et al. To ward the as sess ment of food tox ic ity
for coeliac pa tients: char ac ter iza tion of monoclonal an ti bod ies to a main
immunogenic glu ten pep tide. PLoS ONE. 2008A; May 28; 3(5): e2294.
3. Morón B, Cebolla A, Manyani H, et al. Sensitive detection of cereal fractions
that are toxic to coeliac dis ease pa tients by us ing monoclonal an ti bod ies to a
main immunogenic wheat pep tide. Am J Clin Nutr 2008b; 87: 405-414.
Analytical tools to detect gluten immunotoxic
fractions in food based on monoclonal
antibodies raised against the gliadin 33-mer
peptide
Catherine Torgler 1, Miguel An gel Síglez1, Felipe Vilchez 1, An gel Cebolla1,
Carolina Sousa 2
1 Biomedal S. L., Sevilla, Spain
2 Universidad de Sevilla, Departamento de Microbiología y Parasitología,
Facultad de Farmacia, Sevilla, Spain
Introduction
Immunotoxic gluten peptides that are recalcitrant to degradation of digestive enzymes
appear to trigger coeliac disease (CD). A 33-mer peptide from a-2 gliadin has been
identified as a principal contributor to gluten immunotoxicity [1]. A gluten-free diet is
the unique current therapy for CD patients; therefore, the characterization and
quantification of the toxic portion of gluten in foodstuffs is crucial to avoid coeliac
damage. Our aim was to develop immunological assays as novel food analysis tools to
measure cereal fractions that are immunotoxic to CD patients.
Two monoclonal antibodies (mAb), G12 and A1, were developed against a highly
immunotoxic gliadin 33-mer peptide [2]. In comparison to other ELISAs, those based
on these antibodies showed a wider specificity for prolamins that are toxic to CD
patients, along with a higher degree of sensitivity, accuracy, and reproducibility, than
did the other ELISAs. Analyses of the available prolamin sequences revealed the
potential epitopes in the immunotoxic prolamins of rye, wheat and barley [3]. Although
G12 affinity for the 33-mer was superior to A1, the sensitivity for gluten detection was
higher for A1. This observation correlated to the higher number of A1 epitopes found in
prolamins than G12 epitopes. Both antibodies have been evaluated as analytical tools to
develop different analytical techniques, including ELISA (competitive and sandwich)
and immunochromatographic sticks. To satisfy the increasing demand from CD
patients or their relatives and other potential non-specialized food-related professionals,
we also developed a user-friendly immunochromatographic “sticks” version, called
GlutenTox Home, with G12 mAbs showing consistent results compared to laboratory
techniques for a broad range of food products.
Material and methods
All methods were used according to the manufacturer’s instruction manual (Biomedal
S.L. - GlutenTox ELISA Competitive [ref. KT-4758], GlutenTox ELISA Sandwich
[ref. KT-5196], GlutenTox Sticks [ref. KT-4711]; Ingenasa S.L. - Ingezim Gluten
Assay I-30.GLU.K2; R-Biopharm - RidaQuick Gliadin R7003). For the user-friendly
gluten detection method (GlutenTox Home), the protocol is a simplified version of the
GlutenTox Sticks instructions.
Food sample were ground with a clean food grinder, knife or hammer. With a
graduated plastic spoon (1 mL), two level spoons of ground food was added to a bottle
containing 10 mL of extraction solution (60% EtOH). For liquid samples, only one
spoon (1 mL) was sufficient. For gluten extraction, the tube containing the sample was
shaken vigorously for a total of 1min, then settled for 5 min to allow the solid rest to
sink to the bottom of the tube. Using a platic pipette, a few drops were taken out from
the upper extracted solution. Eight drops to detect 20 ppm were added to a tube
containing 2 mL of dilution solution (1x PBT) The tube was mixed softly and 5 to 6
drops were added to a well at the tip of the immunochromatographic stick encased in a
plastic cassette. After 10 min, the result was read. When a blue control line and a pink
line appeared, the result was positive and above the chosen determined threshold
(20 ppm, Codex Alimentarius norms). When a single blue line appeared, the result
was negative and below 20 ppm and suitable for consumption by CD patients. The
results were then compared with the results from an ELISA Sandwich G12.
Results and discussion
Comparison of R5 and G12 analytical techniques
Several hundreds food analyses were performed to compare G12-based analytical
tools (ELISA Competitive as well as immunochromatographic sticks) to R5 antibodyrelated
techniques. Our results showed concordance in the detection of gluten free
food (< 20 ppm) in > 85% of the analyzed food from external analytical services as
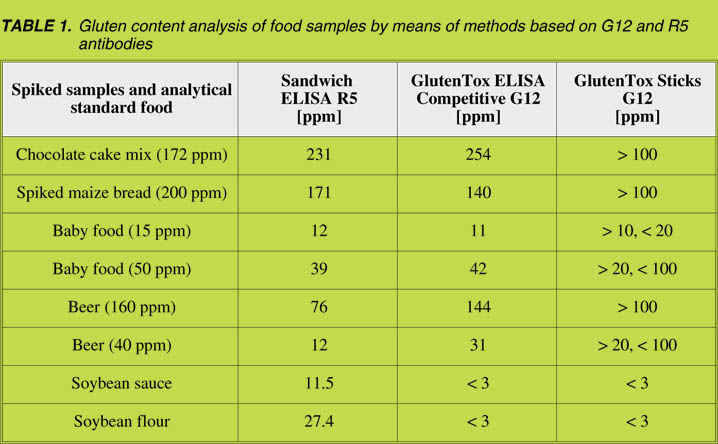
well as spiked samples (data not shown). However, certain discrepancies were found,
and some of them are shown in Table 1. The main discrepancies were found in beer,
probably because the ELISA Sandwich R5 could underestimate immunotoxic gluten
peptides due to the abundance of single epitopes, which cannot be detected by a sandwich
ELISA, although this is feasible by means of the ELISA competitive methods [4].
We detected two food samples containing soybean with no gluten-containing cereals
in the ingredients list, that gave noticeable signals with R5 (Table 1). We also
demonstrated, via different spiked samples, that the immunochromatographic sticks
could consistently estimate gliadin content with different matrices when the dilution
of extracted samples was adjusted (see examples in Table 1).
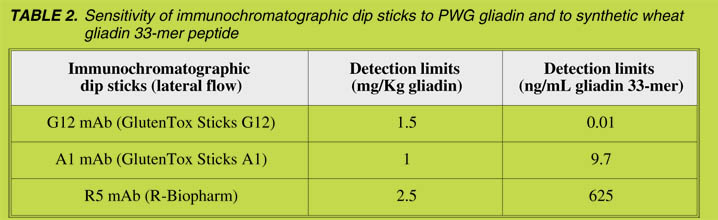
We also tested the capacity of different immunochromatographic sticks with either G12
or A1 antibodies to detect gliadin as well as the main immunotoxic peptide, the gliadin
33-mer. The immunochromatographic sticks with R5, A1 and G12 showed equivalent
sensitivity in detecting gliadin (Table 2). However, R5 showed poor sensitivity in
detecting 33-mer epitopes, since the detection limit was from 62 to 60,000-fold less
sensitive than the A1 and G12 sticks, respectively. Equivalent differences were found
by using ELISA methods (data not shown). These observations may be of particular
relevance for hydrolyzed gliadin since the R5 may underestimate the presence of
immunotoxic peptides.
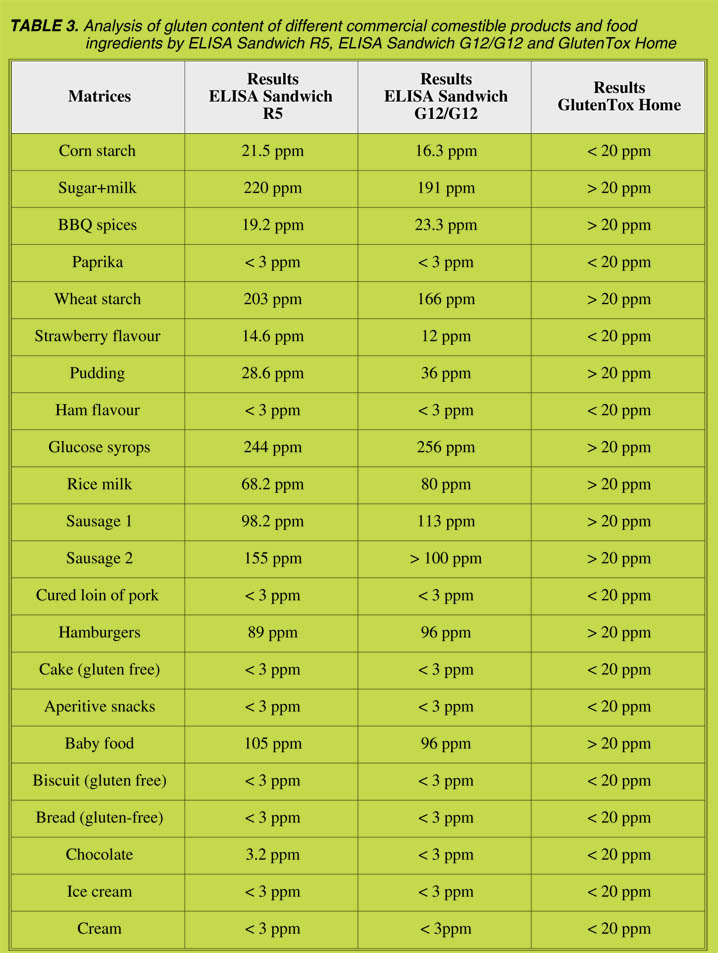
The robustness and the sensitivity of the immunochromatographic sticks encouraged us
to develop a user-friendly kit for gluten detection in food (GlutenTox Home) without
laboratory equipment. Various food samples with different types of matrices were
selected for this study to assess whether a shorter and user-friendly method was
satisfactory for estimating gluten content. Most of the results of this study revealed that
despite the simplicity of the method, the consistency was high, with no discrepancies in
a variety of food matrices (Table 3).
Conclusions
Our study suggests that mAb G12 and A1-based immunotechniques are robust and
sensitive methods to evaluate the potential immunotoxicity of gluten in all types of
food matrices that were tested. The R5-based products showed that they were at least
two orders of magnitude less sensitive to the gliadin 33-mer peptide than G12 or
A1-based methods. The user-friendly lateral flow test (GlutenTox Home), using the
anti-gliadin 33-mer antibody, demonstrated that it could be useful for reliable gluten
content estimations in a variety of food samples despite the simplicity and rapidity of
the protocol.
References
1. Shan L, Molberg Ø, Par rot I, et al. Struc tural ba sis for glu ten in tol er ance in
celiac sprue. Science 2002; 297: 2275-2279.
2. Morón B, Cebolla A, Manyani H, et al. Sensitive detection of cereal fractions
that are toxic to ce liac dis ease pa tients by us ing monoclonal an ti bod ies to a
main immunogenic wheat pep tide. Am J Clin Nutr 2008a; 87: 405-414.
3. Morón B, Bethune M, Comino I, et al. To ward the as sess ment of food tox ic ity
for celiac patients: characterization of monoclonal antibod ies to a main
immunogenic glu ten pep tide. PloS One 2008b; 3: e2294.
4. Dostalek P, Hochel I, Mendez E, et al. Im mu no chemi cal de ter mi na tion of
glu ten in malts and beers. Food Addit Contam 2006; 23: 1074-1078.
II. Clinical research reports
In-vivo quinoa feeding study
Vic tor F. Zevallos1, Su zanne Don nel ly 1, Fuju Chang 2, L. Irene Herencia 3,
H. Julia Ellis1, Paul J. Ciclitira1
1 King's College London, Division of Diabetes and Nutritional Sciences,
De part ment of Gastroenterology, St Thomas’ Hos pi tal, Lon don, UK/England
2 De part ment of Histopathology, St Thomas’ Hos pi tal, King’s Col lege
London, London, UK/England
3 Departamento de Producción Veg e tal, Universidad Politécnica de
Madrid, Spain
Introduction
Dietary gluten can cause inflammation and histological deterioration of the small
intestine in genetically predisposed individuals. An effective treatment for coeliac
patients is to follow a strict gluten-free diet (GFD), however, gluten-free cereals are not
widely available, are less palatable than other foods and can contain fewer nutrients than
their gluten-containing counterparts. New gluten-free products that improve any of
those qualities are a welcome alternative in the GFD. However, it is possible that traces
of immunostimulatory peptides within the prolamin fraction of such alternative
products can exacerbate coeliac disease (CD). Guidance with regard to potential
toxicity can be sought in taxonomic classification and in-vitro experiments but,
ultimately, feeding studies should confirm their suitability.
Quinoa is an Andean crop with excellent nutritional value and balanced amino acid
content, and contains high levels of protein, fibre, vitamins and minerals in comparison
to gluten-containing cereals. However, quinoa cultivars are known to contain up to 7%
prolamin. Those cultivars with the highest content of putative immunostimulatory
prolamins have been identified using in-vitro methods [1] . All cultivars had gluten
levels within the recommended levels (below 20 mg/kg).
Confirmation about their suitability for coeliac patients is needed from an in-vivo
study. Thus the aim of the present study was to examine the clinical, histological and
immunological responses of adult coeliac patients before and after consuming quinoa
as part of their usual GFD.
Materials and methods
Nineteen coeliac patients participated in the study: 2 males and 17 females with a
median age of 59 years and a BMI of 23 kg/m2, who were on a GFD for nine years and
were all HLA-DQ2 positive. The study was approved by the Ethical Committee at St Thomas’ Hospital, London, and all patients gave written informed consent before
participating in the study. All participants were diagnosed adult coeliac patients on a
GFD for at least one year. Participants were excluded if they had any medical condition
considered sufficiently serious to interfere with the study or to constitute an unacceptable
risk to them.
Participants were asked to consume 50 g of pre-weighted quinoa every day for six
weeks as part of their usual gluten-free diet. They were free to choose the cooking
method but it was recommended that they consumed quinoa flakes for breakfast as
porridge or pancakes. Patients were given a diary card [2] to record any symptoms of
diarrhoea, abdominal pain, increase in bowel sounds or vomiting during the entire study
period. Serological coeliac screening tests, including IgA anti-gliadin (AGA), IgA
anti-TTG (AtTG) and IgG and IgA anti-endomysium (EMA), were used to monitor
compliance with the GFD. A full blood count, and liver and renal profiles were used to
monitor the health status of patients. Iron, tests of vitamin B12, serum folate and a lipid
profile were used to determine the effects of quinoa on the GFD. All tests were analysed
before and after the study.
Ten treated coeliac patients provided duodenal biopsies for morphometric measurements
at the beginning and end of the study. The normal range for these parameters are
between 5:1 and 3:1 for VH:CD, between 29 to 34 nm for SECH and between 10% and
30% for the IEL count. CD can be diagnosed when VH:CD is less than 3:1, SECH is
below 29 nm and IEL above 30%. Alterations in these parameters can be used as a
reliable indicator of exacerbation of the condition. The VH:CD ratio was measured in
10 different areas with at least 10 measurements of this ratio per biopsy on H&E stained
slides; the SECH in at least 30 randomly-selected enterocytes in the mid-third of villi per
biopsy on H&E stained slides and the IEL in 10 different areas per biopsy stained for
CD3+ cells.
Results
Gastrointestinal symptoms were differentiated according to four categories (diarrhoea,
abdominal pain, increased bowel sounds and vomiting) and graded daily (0 = none,
1 = mild, 2 = moderate and 3 = severe). Ten patients did not report any symptoms. Nine
patients reported symptoms ranging from mild to moderate during the first two weeks of
the study. Most of them were mild abdominal pain, followed by a mild increase in bowel
sounds and diarrhoea. This might be due to an increase in dietary fibre, as reported in
other feeding studies [3]. Serological coeliac screening test results were within normal
levels.
Duodenal biopsies from 10 patients were assessed randomly and blindly, before and
after consumption of quinoa, by applying three morphometric parameters (VH:CD,
SECH and IEL). Results indicate that the mean values of VH:CD rose from slightly below-normal levels (2.8:1) to normal levels (3:1), similar results were observed for
SECH, with values rising from 28.76 to 29.77 μm. IEL values decreased from slightly
abnormal (30.3) to just below normal (29.7). Although a positive trend was observed
(increased VH:CD and SECH, decreased IEL), no significant differences were seen in
any of the measurements.
The mean values of VH:CD (3:1) and SECH (29.77 μm) at the end of the study were at
the lower end of the normal range (3:1 to 5:1 and 29 to 34 μm, respectively) which was
to be expected in a group of coeliac patients with a wide range of time on a GFD (1 to
33 years) as it could take more than two years to achieve normal or quasi-normal
morphometric parameters after initiation of treatment (GFD) [4]. In some patients,
full recovery is never achieved for various reasons, including hidden sources of gluten
in their diets, complication of the disease (development of refractory CD) or other
unexplained causes [5].
The other morphometric parameter, IEL count, was, conversely, on the higher end of
normality (10% to 30%) after quinoa consumption (29.7%). All median values for
blood tests at the beginning and at end of the study were within the appropriate normal
range, expecting for total cholesterol and LDL, the values for which were slightly
higher than the recommended 4 and 2 mmol/L, respectively [6]. Untreated coeliac
patients tend to have lower cholesterol levels [7] which, after treatment with a GFD,
tend to increase. One of the mechanisms that could contribute to this increment is the
increased absorption of saturated dietary fat after a GFD is started [8].
The total cholesterol in the study population reduced from 4.6 to 4.3 mmol/L, and
LDL fell from 2.46 to 2.45mmol/L, while the reduction in HDL from 1.8 to 1.68
mmol/L was significant (p = 0.05) after eating quinoa. This reduction in cholesterol
confirms the results of an early study in which induced hypercholesterolemia in mice
improved strongly by feeding them with quinoa [9]. Although, the cholesterol values
were still slightly higher than the recommended level and there was a reduction in
HDL, it is clear that patients could benefit from eating quinoa. However, more studies
are needed to determine whether this positive trend continues over a longer period of
time.
Conclusions
The addition of quinoa to the GFD of 19 adult coeliac patients did not cause exacerbation
of the disease. Gastrointestinal symptoms were either absent during the study
or mild in some patients in the first two weeks of the study. Most patients continued
eating quinoa after the study. This could be interpreted as an early indication that
quinoa is well tolerated among coeliac patients. However, a larger number of participants
and a validated method to assess psychological well-being as well as a wider
range of gastrointestinal symptoms would be required to confirm this interpretation.
In addition, the positive trend towards improvements in some serological parameters,
particularly the hypocholesterolemic effects, require further evaluation.
Overall, the data suggest the likely suitability of quinoa as part of a GFD, which had
hitherto been assumed, without supporting clinical data.
References
1. Zevallos VF, Ciclitira PJ, Suligoj T, et al. In vi tro safety as sess ment of quinoa
(Chenopodium quinoa Willd.) in coeliac Dis ease. In: Stern M, ed. Pro ceed ings
of the 22nd Meet ing of the Work ing Group on Prolamin Anal y sis and Tox ic ity,
Sep tem ber 27-29, 2007, Dub lin, Ire land. Zwickau: Verlag Wissenschaftliche
Scripten 2008; 95-98.
2. Ciclitira PJ, Cerio R, Ellis HJ, et al. Eval u a tion of gliadin-con tain ing glu tenfree
prod uct in coeliac pa tients. Hu man Nutrition: Clinical Nutrition 1985; 39:
303-308.
3. Storsrud S, Olsson M, Arvidsson Lenner R, et al. Adult coeliac pa tients do
tol er ate large amounts of oats. Eur J Clin Nutr 2003; 57: 163-169.
4. Wahab PJ, Meijer JWR, Mulder CJJ. Histologic fol low-up of Peo ple with
ce liac dis ease on a glu ten-free diet. Am J Clin Pathol 2002; 118: 459-463.
5. Lee SK, Winson L, Lorenzo M, et al. Du o de nal his tol ogy in pa tients with
ce liac dis ease af ter treat ment with a glu ten-free diet. Gastrointest Endosc 2003;
57:187-191.
6. NICE clinical guideline 67. Lipid modifica tion, National In stitute for Health
and Clin i cal Ex cel lence, 2008 (re is sued 2010).
7. West J, Lo gan RFA, Hill PG, et al. Seroprevalence, cor re lates, and characteristics
of un de tected coeliac dis ease in Eng land. Gut 2003; 52: 960-965.
8. Brar P, Kwon YG, Hollen S, et al. Change in lipid pro file in ce liac dis ease:
Beneficial effect of gluten-free diet. Am J Med 2006; 119: 790.
9. Konishi Y, Arai N, Umeda J, et al. Cho les terol low er ing ef fect of the meth a nol
in sol u ble ma te ri als from the quinoa seed pericarp. In: Katsuyoshi N, ed. Hy drocolloids.
Am ster dam: Elsevier Sci ence 2000; 417-422.
Immunogenicity of two oats varieties
Mariantonia Maglio1, Giuseppe Mazzarella 2, Maria Vit toria Barone1,
Carmen Gianfrani 2, Norberto Pogna3, Rosita Stefanile 2,
Alessandra Camarca 2, Barbara Colicchio1, Merlyn Nanayakkara1,
Salvatore Auricchio1, Riccardo Troncone1
1 FID European Laboratory for the Investigation of Food Induced Dis eases,
Uni ver sity Federico II, Naples, It aly
2 In sti tute of Food Sciences CNR, Avellino, It aly
3 Unità di Ricerca per la Valorizzazione qualitativa dei cereali, Rome, Italy
Introduction
Coeliac disease (CD) is characterised by a derangement of both the adaptive and the
innate immune response to gliadin. Some gliadin peptides that are deamidated by tissue
transglutaminase (e.g., A -gliadin P57-68) bind to HLA DQ2 and/or DQ8 molecules and
induce an adaptive Th1 proinflammatory response [1]. Other gliadin peptides (e.g.,
P31-43, P31-49) are not recognized by T cells and induce an innate immune response
mainly mediated by IL15 [2].
To date there is still little information on the immunogenic properties of cereals others
than wheat. Protein fractions of barley and rye, which are known to be toxic to coeliac
patients, are able to activate gliadin-reactive T-cell lines obtained from the intestine of
coeliac patients, showing that these cereals, like wheat, can induce an adaptive immune
response [3]. Nothing is known regarding the ability of these cereals to induce an innate
immune response.
Recently, several studies have focused on oat as a cereal that can be introduced into the
CD diet. In-vivo studies in children and adults seem to indicate that oats can be tolerated
by CD patients [4]. However, Lundin et al. [5] reported that some patients on an oatcontaining
diet had abdominal discomfort and one patient developed villous atrophy
and dermatitis. Moreover it has been shown that gliadin-reactive T-cell lines obtained
from the intestine of coeliac patients may respond to protein fractions of oats [3].
Although oats have been the object of several studies in the recent times, some
questions about their toxicity still remain unanswered. One issue is the individual
reactivity of CD patients, as some CD patients are responsive to oats. Another issue is
the possibly different toxicity of oats varieties.
The aim of our study was to investigate immunological properties of two oats
varieties, Avena genziana and Avena potenza, in terms of their safety for coeliac
patients.
Methods and results
IL15 expression
High levels of IL-15 are present in intestinal mucosa of coeliac patients in the active
phase of the disease. By means of immunohistochemistry, we investigated the IL15
expression both in the epithelium and lamina propria of the intestinal mucosa of CD
patients on a gluten-free diet (GFD) before and after 24 hours of in-vitro treatment
with the medium alone or with PTG, PT-genziana or PT-potenza. Before culture, IL15
was highly expressed in the villi and crypts epithelium of small intestinal mucosa of
CD patients on a GFD. The cytokine was mainly found in the apical region of the
enterocytes. This pattern sometimes presented as a patchy distribution. IL15-positive
cells were also detected in the lamina propria.
After in-vitro culture for 24 hours with the medium alone, IL15 staining decreased
both in the villi and crypts epithelium. In-vitro culture in the presence of PTG, induced
an increase in IL15 expression in villi and crypts epithelium and in the lamina propria
as against tissue cultured with the medium alone. On the contrary, after 24 hours of
culture with PT-genziana and PT-potenza, we did not observe any significant increase
in IL15 staining in villi and crypts epithelium or in the lamina propria.
Intraepithelial lymphocytes infiltration
A significant increase of CD3+ intraepithelial lymphocytes was seen in biopsies of
CD patients on a GFD, which were cultured for 24 hours in the presence of PTG (32± 18 cell/mm epithelium) and also with PT-potenza (23 ± 12 cell/mm epithelium), as
against those cultured in medium alone (13 ± 6 cell/mm epithelium). By contrast, no
differences were noted in the number of intraepithelial CD3+ cells in biopsy specimens
treated with PT-genziana (16 ± 8 cell/mm epithelium).
Mononuclear cell activation in lamina propria
CD25 expression in the lamina propria was evaluated to find evidence of activated
mononuclear cells. Consistent with previous results, the expression of the abovementioned
marker was significantly higher in biopsy specimens from CD patients on
a GFD after 24 hours in-vitro treatment with PTG (49 ± 32 mm2 of lamina propria) in
comparison to the tissue cultured using the medium alone (13 ± 8 CD25+/mm2 of
lamina propria). Such an increase was not observed in fragments cultured with
PT-genziana (23 ± 17 CD25+/mm2 of lamina propria) or PT-potenza (21 ± 17
CD25+/mm2 of lamina propria).
T-cell lines and IFNg production
We analyzed the long-term ability of prolamins from the oat varieties potenza and
genziana to stimulate intestinal T-cells lines established from eight DQ2 positive coeliac
individuals and raised against deamidated PT-gliadin. All iTCLs displayed marked IFNg
production when stimulated with deamidated PT-gliadin: median: 5.7 ng/ml (range
0.9-12.5). Although iTCLs from 3 patients cross-reacted with PT-genziana, and only
when it was deamidated, on the whole the IFNg induced by the oat genziana was much
lower than the level observed in responses to gliadin: 1.9 ng/ml (range 0-10) (p < 0.05).
With the exception of one patient, in which a positive response to PT-potenza was
elicited, with an average of IFNg production of 1.4 ± 10 ng/ml, PT-potenza did not induce
significant response in any of the patients analyzed.
Conclusions
In this study, several in-vitro tests and biological assays were used to investigate the
immunological effects of two oat varieties, Avena genziana and Avena potenza, in CD
patients. These tests were designed to investigate the adaptive and the innate immune
response of the coeliac intestine to oats prolamins in comparison to gliadin peptides.
Oats prolamine peptides were not able to induce, in CD enterocytes, an increase in
IL15 or in D25 positive cells. Overall, the results of the in-vitro tests suggest that the
two oats varieties we studied are virtually safe for CD patients. These results are
consistent with those of clinical studies demonstrating that oats are generally well
tolerated by the majority of CD patients [4, 6]. We propose this experimental
approach, based on an extensive in-vitro evaluation of biological and immunological
proprieties, to screen cereal varieties selected by breeding or modified for coeliac
patients, before conducting in-vivo studies.
References
1. Camarca A, An der son RP, Mamone G, et al. In testinal T-cell responses to
glu ten pep tides are largely het er o ge neous: im pli ca tion for a pep tide-based
ther apy in ce liac dis ease. J Immunol 2009; 182: 4158-4166.
2. Maiuri L, Ciacci C, Ricciardelli I, et al. As so ci a tion be tween in nate re sponse to
gliadin and ac ti va tion of patho genic T cells in coeliac dis ease. Lan cet 2003;
362: 30-37.
3. Kilmartin C, Wieser H, Abuzakouk M, et al. In tes ti nal T cell re sponses to
ce real pro teins in ce liac dis ease. Dig Dis Sci 2006; 51: 202-209.
4. Pulido OM, Gillespie Z, Zarkadas M, et al. In tro duc tion of oats in the diet of
individ uals with celiac disease: a sys tematic review. Adv Food Nutr Res 2009;
57: 235-285.
5. Lundin KEA, Nilsen EM, Scott HG, et al. Oats in duced villous at ro phy in
coeliac dis ease. Gut 2003; 52: 1649-1652.
6. Kilmartin C, Lynch S, Abuzakouk M, et al. Avenin fails to in duce a Th1
re sponse in coeliac tis sue fol low ing in vi tro cul ture. Gut 2003; 52: 47-52.
Gluten toxicity, how to get rid of it?
Frits Koning1, Rene Smulders 2
1 De part ment of Immunohematology and Blood Trans fu sion,
Leiden Uni ver sity Med i cal Cen tre, Leiden, The Neth er lands
2 Plant Re search In ter na tional, Wageningen UR, The Neth er lands
In 1993 Lundin and colleagues first described the presence of gluten-specific T cells in
small intestinal biopsies of coeliac disease (CD) patients [1]. A large number of studies
have since established that such T cells can be specific for a large and diverse array of
peptides derived from the gliadins as well as the glutenins ([2-5], references in [6]).
Invariably, these peptides can trigger T-cell responses only when bound to the disease
predisposing HLA-DQ2 or HLA-DQ8 molecules. This provides an explanation for the
well established association between these HLA-molecules and disease development. It
also became evident that many of these peptides require modification by the enzyme
tissue transglutaminase, a modification that introduces negative charges into gluten
peptides, thus enhancing the binding of these peptides to either HLA-DQ2 or HLADQ8
[8]. Not only wheat is off-limits to CD patients: barley and rye are also known to
contain a variety of proteins that are just as harmful as the gluten proteins from wheat
[8, 9]. Oat seems an exception as it is tolerated by most patients, partly due to a low
content of gluten-like prolamin proteins [8-10].
Thus, CD patients usually have T cells specific for an array of gluten and gluten-like
peptides that originate from all types of gluten proteins and homologues in other cereals.
At present the introduction of a gluten-free diet is the only but highly-effective treatment
option. This diet, however, has several drawbacks. It is relatively cumbersome, difficult
to adhere to, expensive and is deficient in several nutrients and fibers. Many patients feel
insecure, especially when eating out or while travelling. Thus, there is an unmet need for
alternatives to the gluten-free diet.
With the identification of the harmful sequences in gluten and gluten-like proteins it has
become possible to initiate studies aimed at reducing or eliminating the toxicity of such
proteins and/or wheat. Early studies indicated that substantial differences existed
between wheat varieties regarding their "toxicity profile" [11-13]. Some of those could
be attributed to differences in the genetic make-up of the three genomes that comprise
the complex hexaploid bread wheat, the A-, B- and D-genome [12, 13]. More recently
we embarked on a large-scale study to map the toxicity of the a-gliadins, based on the
observation that the a-gliadins are among the most immunogenic gluten proteins and contain four well-characterized peptides involved in CD [2, 4, 5]. We analyzed over
3,000 a-gliadin genes in the database to determine the full extent of the natural
variability that is present in these genes and in the known T-cell stimulatory peptides in
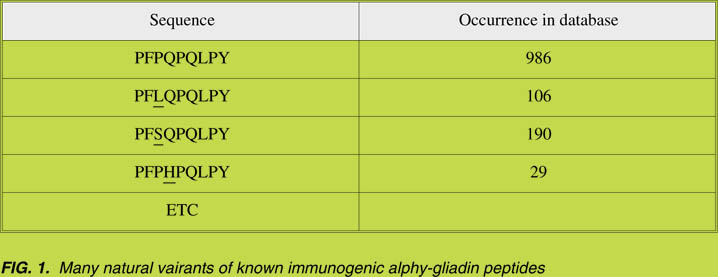
these proteins in particular [14]. The results indicated that many natural variants of these
immunogenic a-gliadin peptides exist; an example is given in Fig. 1.
We could classify all identified variants as belonging to one of the three genomes
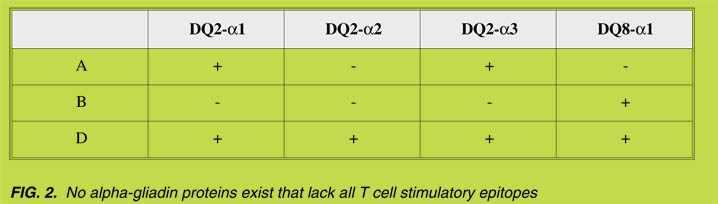
based on differences in the complete gene sequences. Moreover we synthesized all
variants of the four known T-cell peptides that we had identified, and tested these for
binding to HLA-DQ2 or HLA-DQ8 as well as for recognition by T cells derived from
small intestinal biopsies of CD patients ([14], Fig. 2). The results demonstrated that no
a-gliadin proteins exist that lack all T-cell stimulatory epitopes ([14], Fig. 2). Based
on these results it can be concluded that it would be impossible to generate CD-safe
wheat through conventional breeding programs. Substantial differences, however,
were observed between the genes encoded by the three genomes: while the D-genome
a-gliadins are by far the most toxic, the B-genome encoded a-gliadins are the least
toxic and the A-genome encoded genes have an intermediate toxicity profile.
Close examination of the results, however, indicated that there are natural variants of
all T-cell stimulatory epitopes that do not induce T-cell responses. For example, the
A-genome encodes a variant of the DQ2-a2 epitope that is not immunogenic: while
the sequence of this epitope on the D-genome is PQPQLPYPQ, the A-genome
encodes PQPQLPYSQ and this P to S substitution results in a peptide that does not
induce T-cell responses [14]. Similarly, natural variants of the other three T-cell
stimulatory peptides have been identified that lack T-cell stimulatory properties.
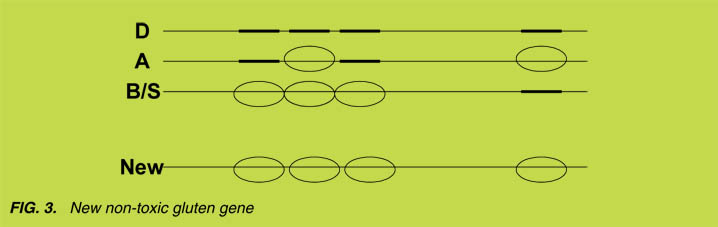
These results allow a novel approach to eliminate gluten toxicity: while the D-genome
a-gliadin gene encodes four toxic epitopes, it is possible, , by combining the genetic
information of the A- and B-genome encoded a-gliadins, to generate a new gene that
encodes a that is not toxic for CD patients ([14], Fig. 3). At the peptide level, we have
provided proof of principle for this approach and we envisage that similar approaches
can be taken to generate non-toxic variants for the other gliadin and glutenin proteins in
wheat gluten. In this respect the high molecular weight glutenins are of particular
interest as these, to a large extent, determine the baking properties of gluten. Genes
encoding such safe-gluten proteins could be introduced into safe cereals for production
of safe-gluten that could be used for the fabrication of gluten-free foods with markedly
enhanced quality in terms of nutritional value, taste and baking properties.
Acknowledgement
This research was financed in part by the Celiac Disease Consortium, an Innovative
Cluster approved by the Dutch Genomics Initiative and partially funded by the Dutch
Government (BSIK03009).
References
1. Lundin KE, Scott H, Hansen T, et al. Gliadin-spe cific, HLA-DQ(a1*0501,b1*0201)
re stricted T cells iso lated from the small in tes ti nal mu cosa of ce liac dis ease
patients. J Exp Med 1993; 178: 187-196.
2. Van de Wal Y, Kooy Y, van Veelen P, et al. Small in tes ti nal cells of ce liac
disease patients recognize a natural pepsin fragment of gliadin. Proc Natl Acad
Sci USA 1998; 95: 10050-10054.
3. Van de Wal Y, Kooy YMC, van Veelen P, et al. Glutenin is in volved in the
glu ten-driven mucosal T cell re sponse. Eur J Immunol 1999; 29: 3133-3139.
4. Arentz-Hansen H., Körner R., Molberg Ø, et al. The in tes ti nal T cell re sponse to
a-gliadin in adult ce liac dis ease is fo cused on a sin gle deamidated glutamine
tar geted by tis sue transglutaminase. J Exp Med 200; 191: 603-612.
5. Vader W, Kooy Y, van Veelen P, et al. The glu ten re sponse in chil dren with
re cent on set ce liac dis ease. A highly di verse re sponse to wards mul ti ple gliadin
and glutenin de rived pep tides. Gastroenterology 2002; 122: 1729-1737.
6. Stepniak D, Koning F. Ce liac Dis ease: sandwiched be tween in nate and adap tive
immu nity. Hum Immunol 2006; 67: 460-468.
7. Van de Wal Y, Kooy Y, van Veelen P, et al. Cut ting Edge: Se lec tive deamidation
by tis sue transglutaminase strongly en hances gliadin-spe cific T cell
reactiv ity. J Immunol 1998; 161: 1585-1588.
8. Vader W, de Ru A, van de Wal Y, et al. Specificity of tissue transglutaminase
explains cereal toxicity in celiac disease. J Exp Med 2002; 195: 643-649.
9. Vader W, Stepniak D, Bunnik EM, et al. Characterization of cereal toxicity for
ce liac dis ease pa tients based on pro tein homology in grains. Gastroenterology
2003; 125: 1105-1113.
10. Arentz-Hansen H, Fleckenstein B, Molberg Ø, et al. The mo lec u lar ba sis for oat
intolerance in patients with celiac disease. PLoS Medi cine 2004; 1: 84-92.
11. Spaenij-Dekking L, Kooy-Winkelaar Y, van Veelen P, et al. Natu ral variation in
toxicity of wheat accessions for celiac disease patients. Potential for selection and
breed ing of non-toxic wheat va ri et ies. Gastroenterology 2005; 129: 797-806.
12. Molberg Ø, Uhlen AK, Jensen T, et al. Map ping of glu ten T-cell epitopes in the
bread wheat ancestors: implications for celiac disease. Gastroenterology 2005;
128: 393-401.
13. Van Herpen TWJM, Goryunova SV, van der Schoot J, et al. Al pha-gliadin genes
from the A, B, and D genomes of wheat con tain dif fer ent sets of ce liac dis ease
epitopes. BMC Genomics 2006; 7: 1.Mitea C, Salentijn EMJ, van Veelen P, et al.
A univer sal approach to eliminate anti genic properties of alpha-gliadin peptides
in ce liac dis ease. PLoS One 2010; 5: e15637.
14. Mitea C, Salentijn EMJ, van Veelen P, et al. A uni ver sal ap proach to elim i nate
anti genic properties of alpha-gliadin peptides in celiac disease. PLoS One 210; 5:
e15637.
Antibodies in the diagnosis of coeliac
disease in young children
Thomas Rich ter 1, Xa vier Bossuyt 2, Pieter Vermeersch 2, Holm Uhlig 3,
Sybille Koletzko4, Klaus-Pe ter Zimmer 5, Cornelia Dähnrich6,
Thomas Mothes 7
1 Mu nic i pal Hos pi tal "Sankt Georg" Leip zig, Ger many
2 Dept. Lab o ra tory Med i cine of Uni ver sity Hos pi tal Leuven, Bel gium
3 Uni ver sity Chil dren's Hos pi tal Leip zig, Ger many
4 Uni ver sity Chil dren's Hos pi tal, Mu nich, Ger many
5 Uni ver sity Chil dren's Hos pi tal Giessen, Ger many
6 Euroimmun Medizinische Labordiagnostika GmbH Lübeck, Ger many
7 In sti tute of Lab o ra tory Med i cine, Clin i cal Chem is try and Mo lec u lar
Di ag nos tics, Uni ver sity Hos pi tal Leip zig, Ger many
Introduction
Assays meassuring IgA antibodies against tissue transglutaminase (anti-tTG) and
endomysium (EmA) and IgG antibodies against deamidated gliadin peptides in serum
have a high sensitivity and specificity for coeliac disease (CD) in children [1, 2].
However, in young children (up to two years of age), antibodies against native gliadin
(anti-nGli) are still assumed to have a higher diagnostic accuracy.
In young children, IgA-EmA are reportedly less sensitive, and have maximum values
of 89% [3-8]. The sensitivity of IgA-anti-tTG ranged between 83% and 90% [1?, 2?,
6, 8, 9]. The specificity of IgG-anti-nGli was only 77% at high sensitivity [5]. It has
been claimed that IgA-anti-nGli was the best means for detecting CD in young
children [6], as it has a sensitivity between 82% and 97% and a specificity between
88% and 94% [1?, 2?, 5-8]. Little data is available to date on the test performance of
antibodies against deamidated gliadin peptides are rare until now, particularly in very
young children. We investigated the validity of antibodies against deamidated gliadin
peptides and compared the results with those from other antibody tests.
Patients and methods
The sera of 173 children below three years of age were retrospectively examined. The
patients were recruited from the Municipal Hospital "St. Georg" in Leipzig (Germany),
the Departement of Laboratory Medicine of the University Hospital in Leuven
(Belgium), and also from the University Children's Hospital of Leipzig, Munich, and
Giessen (Germany). The patients comprised 39 children with CD and 134 controls (93 females and 80 males, mean age 1.57 years, 95% range 0.6 to 2.9 years). Sera samplings
wa done at the time of duodenal biopsy. All patients following a normal (glutencontaining)
dietand were biopsied to suspicion of CD or other gastrointestinal disorders.
The intestinal pathology of all CD patients was in accordance with Marsh 2 or Marsh 3
criteria.
IgA and IgG antibodies against deamidated gliadin (analogous fusion) peptides
(anti-GAF3X), anti-nGli, anti-tTG, and IgA-EmA were measured (without prior knowledge
of the diagnosis) by means of the test kits from EUROIMMUN Medizinische
Labordiagnostika Lübeck, Germany. The analyses were performed by EUROIMMUN.
Data were evaluated by means of the receiver operating characteristic (ROC) analysis.
The area under the ROC-curves (AUC) was calculated. Differences between ROCcurves
were evaluated by pairwise comparison according to a chi-square analysis. An
error probability of less than 0.05 was considered statistically significant. Noninferiority
testing was performed if there was no statistically-significant difference.
For non-inferiority testing, the lower end of the 90% confidence intervals (CI) of
differences between AUCs was considered. Non-inferiority was assumed if the lower
end of the 90% CI of the difference was not below 0.01 (zone of diagnostic indifference
of 1%).
For the cut-offs suggested by the manufacturer, diagnostic accuracies, sensitivities,
and specificities were calculated. The significance of differences (p < 0.05) was
evaluated by applying McNemar's test in which applying 2 x 2 contingency tables
containing the number of patients are classified as correct or incorrect. For noninferiority
testing, we compared two tests showing the percentage of children with
false-negative, false-positive, or false positive and false-negative results, for the
oeliac patients, the control children, and for all patients, respectively. The z-test was
applied for calculation of the 90% CI of differences in proportions. Non-inferiority
was assumed if the lower end of the 90% CI of the difference in proportions was not
below 0.01.
Results
The results are summarized in Table 1. After examining all the antibody tests, we
found that the AUC of IgG-anti-GAF was the highest (0.960). The AUC of
IgG-anti-GAF was significantly higher than that of IgA-anti-nGli and non-inferior to
that of IgG-anti-nGli, of IgG-anti-tTG and of IgA-EmA. In the case of IgA-anti-GAF,
it was not possible to draw any conclusions interms of significance of differences or
non-inferiority.
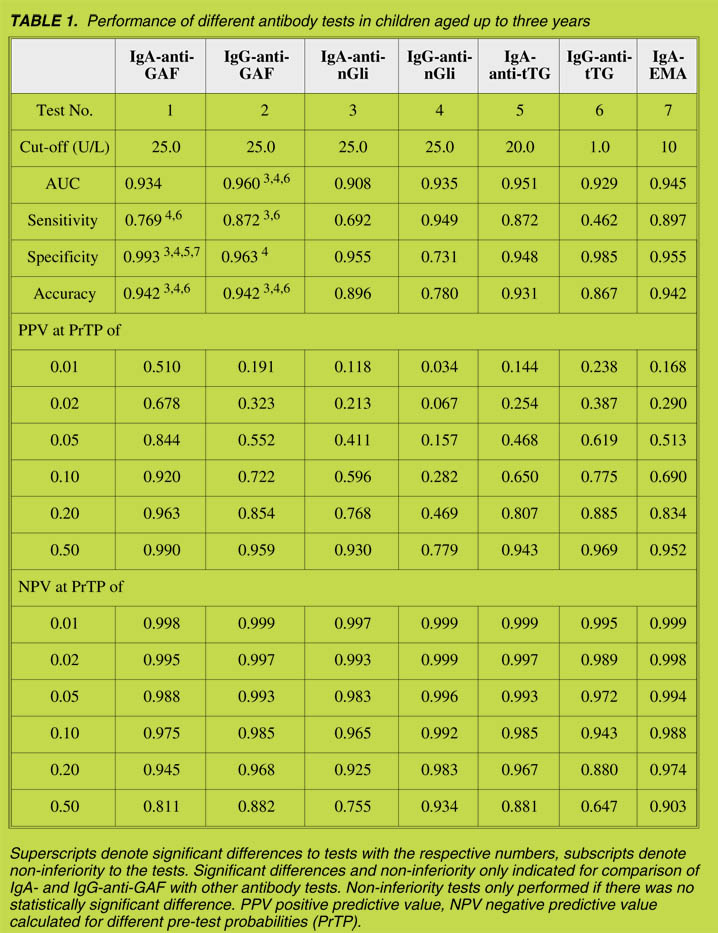
In the analysis in which the manufacturer's cut-off in diagnostic accuracy was found
for IgG-anti-GAF, IgA-anti-GAF and of IgA-EmA was applied, the highest (0.942 in
all three antibody tests). The accuracy of IgG-anti-GAF was significantly higher than
that of IgG-anti-nGli and of IgG-anti-tTG, but non-inferior to IgA-anti-nGli. The
accuracy of IgA-anti-GAF was significantly higher than that of IgG-anti-nGli, IgAanti-
nGli and IgG-anti-tTG. A comparison between IgA- and IgG-anti-GAF yielded
inconclusive results.
The specificity of both anti-GAF-tests was at least non-inferior to the IgG-anti-nGli
assay. Non-inferiority to the IgA-anti-nGli assay could only be shown for the IgAanti-
GAF test, although the estimate of the specificity of both anti-GAF tests was
higher than that of the two anti-nGli assays.
Whereas the sensitivity of the IgG-anti-GAF test was significantly higher that of the
IgA-anti-nGli assay, a comparison with the IgG-anti-nGli assay proved inconclusive.
The sensitivity of the IgA-anti-GAF test was even significantly lower than that of the
IgG-anti-nGli assay and a comparison with the IgA-anti-nGli assay proved inconclusive.
In a pre-test probability of up to 10%, negative predictive values were higher than 0.98
for IgG-anti-GAF, IgG-anti-nGli, IgA-anti-tTG, and IgA-EmA. With increasing pretest
probabilities, negative predictive values for all assays, except that for the IgGanti-
nGli test decreased below 0.93. Positive predictive values were lowest for IgGanti-
nGli, regardless of the pre-test probability.
In one patient, none of the tested antibodies was positive (Marsh 3c). In three other
patients (all Marsh 3a), only IgG-anti-nGli were elevated. In one of these three
patients (HLA-DQ8 positive) secretory IgA deficiency could be demonstrated.
Summary and conclusion
Our study did not only comprise children up to two years of age (as it was described in
previous reports cited above) but also included children in the age group between two
and three years. We included the latter group in order to obtain a sample size sufficient
for statistical evaluation. The inclusion of older children, however, may hide the
special features of antibody diagnostics in the young age group.
Only 23% of the patients studied were coeliacs. Thus, the accuracy is strongly
determined by specificity. The accuracy of both anti-GAF assays was at least noninferior
to that of the two conventional anti-nGli-tests. Both, accuracy and specificity
of the two anti-GAF tests are saytisfactory as they are at least non-inferior to the two
assays for anti-nGli (excepting in the comparison of the IgG-anti-GAF assay with the
IgA-anti-nGli-test, whichwas inconclusive).
We conclude that the measurement of IgG-anti-GAF, IgG-anti-nGli, IgA-anti-tTG,
and IgA-EmA results in the highest negative predictive values in children below three
years of age. In a pre-test probability up to 10%. the negative predictive values of these
tests are at least 98.5%. Anyway these assays, however, the positive predictive value
of the IgG-anti-nGli test is very low, which renders this test unsuitable as a diagnostic
tool.
A selection bias in our results cannot be excluded. First of all, biopsies are more likely
to be performed in symptomatic patients with positive results for CD specific antibodies
such as IgA-EmA and IgA-anti-tTG, which favours these tests. On the other
hand, most children in this study had not been re-challenged with gluten in order to
confirm the diagnosis of CD as per the ESPGHAN criteria [10]. Therefore, we cannot
guarantee that the three patients with enteropathy and positive IgG-anti-nGli, but with
negative results for all other antibody tests, were true CD patients. Since the specificity
of anti-nGli antibodies is low, mucosal lesions due to infections or allergy might
have been taken as indicators of CD. Confirmation or exclusion the diagnosis of CD is
therefore crucial and should be done by means of follow-up, exclusion of IgA
deficiency, HLA-analysis and/or gluten provocation.
Due to the low number of coeliac patients, the power of our study was low. More
patients with clearly defined diagnostic criteria, especially children below two years
of age are required in future studies.
References
1. Prause C, Rich ter T, Koletzko S, et al. New de vel op ments in serodiagnosis of
child hood ce liac dis ease: as say of an ti bod ies against deamidated gliadin.
An nals N Y Acad Sci 2009; 1173: 28-35.
2. Prause C, Ritter M, Probst C, et al. An ti bod ies against deamidated gliadin as
new and ac cu rate biomarkers of child hood coeliac dis ease.
J Pediatr Gastroenterol Nutr 2009; 49: 52-58.
3. Bürgin-Wolff A, Gaze H, Hadziselimovic F, et al. Antigliadin and antiendomysium
an ti body de ter mi na tion for coeliac dis ease. Arch Dis Child 1991; 66:
941-947.
4. Ghedira I, Sghiri R, Ayadi A, et al. Anticorps anti-endomysium, anti-réticuline
et anti-gliadine, intérêt dans le di ag nos tic de la maladie coeliaque chez l'enfant.
Pathol Biol 2001; 49: 47-52.
5. Tonutti E, Visentini D, Bizzaro N, et al. The role of antitissue transglutaminase
as say for the di ag no sis and mon i tor ing of coeliac dis ease: a French-Ital ian
multicentre study. J Clin Pathol 2003; 56; 389-393.
6. Lagerqvist C, Dahlbom I, Hansson T, et al. Antigliadin im mu no glob u lin A best
in find ing ce liac dis ease in chil dren youn ger than 18 months of age.
J Pediatr Gastroenterol Nutr 2008; 47: 428-435.
7. Wolters VM, van de Nadort C, Gerritsen SAM, et al. Is glu ten chal lenge re ally
nec es sary for the di ag no sis of ce liac dis ease in chil dren youn ger than age two
years? J Pediatr Gastroenterol Nutr 2009; 48: 566-570.
8. Maglio M, Tosco A, Paparo F, et al. Se rum and in tes ti nal ce liac dis easeas
so ci ated an ti bod ies in chil dren with ce liac dis ease youn ger than two years
of age. J Pediatr Gastroenterol Nutr 2010; 50: 43-48.
9. Basso D, Guariso G, Fogar P, et al. An ti bod ies against syn thetic deamidated
gliadin pep tides for ce liac dis ease di ag no sis and fol low-up in chil dren.
Clin Chem 2009; 55: 150-157.
10. Walker-Smith JA, Guandalini S, Schmitz J, et al. Re vised cri te ria for di ag no sis
of coeliac dis ease. Re port of Work ing Group of Eu ro pean So ci ety of Pae di at ric
Gastroenterology and Nu tri tion. Arch Dis Child 1990; 65: 909-911.
Signalling pathways controlling the TG2 expression
in the small intestinal mucosa
Mariela Bayardo, Constanza Bondar, Fernando G. Chirdo
Laboratorio de Investigación en el Sistema Inmune - LISIN, Facultad de
Ciencias Exactas, Universidad Nacional de La Plata, La Plata, Argentina
Introduction
Transglutaminase 2 (TG2) is a multifunctional protein located in several cell compartments
such as the cytoplasm, the nuclei and the mitochondria. TG2 works as a G protein
in transmembrane signalling and has protein disulphide isomerase and protein kinase
activity. It is, however, the catalytic activity leading to either deamidation or formation
of e-(g-glutamil) lysine crosslinks between proteins (termed transamidation) that has
received major attention [1]. This biological activity can either occur on the cell surface
or in the extra cellular matrix, where it crosslinks matrix components that promote tissue
stability, cell adhesion and cell migration. Altogether, these functional properties have
important roles in tissue repair, inflammation and apoptosis. The dysregulation of TG2
is involved in the pathogenesis of various human disorders, including neurodegenerative
and autoimmune diseases [2, 3].
Recent research has therefore focussed not only on the role of TG2 in the pathogenic
mechanisms of disease but also on the therapeutic use of its pharmacological inhibition
[4]. Particularly in coeliac disease (CD), selective deamidation of glutamine residues in
gliadin/glutenin-derived peptides leads to higher affinity binding to the HLA predisposing
alleles DQ2 (A1*0501, B1*0201) and DQ8 (A1*0301, B1*0301), thus leading to
greater gliadin-specific T cell stimulation. In addition, TG2 catalyzes a covalent linkage
between the glutamine-rich gliadins/glutenins and itself or with other acceptor proteins
[5].
The small intestinal mucosa in untreated CD patients is characterized by the presence
of several proinflammatory cytokines such as TNFa, gIFN, IL-6, and IL-15, among
others. Although signal transduction pathways of proinflammatory cytokines have
been investigated in different systems, their role in TG2 expression in the intestinal
mucosa has not yet been assessed.
The aim of this study was to evaluate the induction of TG2 expression by proinflammatory
cytokines and to assess the signalling pathways operating in the small
intestine.
Methods
Cell line: Caco-2 cells (human colon adenocarcinoma) were used as a model of human
intestinal epithelia.
Biopsy samples: Intestinal biopsies were taken as part of routine procedure to diagnose
CD in adult patients suffering from different gastrointestinal symptoms. The diagnosis
was reached by means of histological examination, serology and analysis of clinical
presentation. The present study was approved by the HIGA San Martin hospital's ethical
committee, La Plata, Argentina.
Cytokine treatments and inhibition of signalling pathways: Caco-2 cells and biopsy
samples were either stimulated only with proinflammatory cytokines for 24 h (TNFa 10
ng/ml endogen RTNFA1, gIFN 200 UI/ml BD554617, IL-1 10 ng/ml BD 551838, IL-6
10 ng/ml and IL-15 20 ng/ml BD 554630) or in the presence of inhibitors of signalling
pathways, such as JNK/C-Jun pathway (SP600125 20 mM calbiochem), MAPK-p38
pathway (SB203580 10 mM calbiochem), PI3K pathway (wortmanine 10 mM calbiochem),
AKT pathway (Ly294002 2 mM sigma) and NF-kB pathway (sulfasalazine
10 mM sigma and Bay11-7082 1 mM sigma).
Quantitative real-time PCR: Using the sequence of TG2 gene reported by our group
(Genbank AY675221), a specific pair of primers was designed to amplify a 243 bp TG2
fragment. Real-time PCR was performed to determine the TG2 RNAm level after
different treatments. Quantitative PCR was performed in the Cycler real-time PCR
(BioRad). b-actin was the housekeeping gene used for normalisation.
Results and Discussion
In active CD, proinflammatory cytokines, such as TNFa, gIFN, IL-1, IL-6 and IL-15,
are actively produced in the small intestinal mucosa. In-vitro assays were used to
evaluate the role of these cytokines on the modulation of TG2 expression. Caco-2
cells were incubated for 24 h with TNFa, gIFN, IL-1, IL-6 and IL-15 and the
expression of TG2 was determined by quantitative real-time PCR. Figure 1A shows
that gIFN and TNFa are the most potent inducers of TG2 expression; in particular,
gIFN produced an 18-fold increase.
Taking into consideration that gIFN and TNFa are typical Th1 cytokines and
abundant in the small intestine mucosa in active CD, we further evaluated the
expression of TG2 on Caco-2 cells stimulated by both cytokines. The results of the
quantitative PCR showed that the expression of TG2 in Caco-2 cells treated with
TNFa+gIFN was higher (25,7) than the value estimated by adding the fold increase
values for TNFa and gIFN (21,2) (Fig. 1B). Therefore, the incubation of TNFa+gIFN
produced a synergistic effect on TG2 induction.
Quantitative RT-PCR showed that TG2 was strongly induced by TNFa+gIFN, both in
untreated CD patients as well as controls (Fig. 2). The signalling pathways observed in
Caco-2 cells are also active in the small intestine. The induction was much higher in
active CD than in the control samples, suggesting that signalling pathways are
activated to a higher extent in untreated CD patients, due to the chronic inflammatory
process, or, instead, that CD patients tend to respond more strongly to this stimulus.
The TG2 promoter contains binding sites for several transcriptions factors, with
NFkB being one of the most important due to the multiplicity of its biological effects.
A set of inhibitors were used to assess the signalling pathways involved in TG2
induction by TNFa and gIFN. Caco-2 cells were incubated with TNFa, gIFN or
TNFa + gIFN, in the presence of the following inhibitors: SP600125 (C-Jun),
SB20358 (MAPKp38), wortmanine (PI3K), Ly294002 (AKT), sulfosalazine (NFkB)
or Bay 11-7082 (NFkB).
When Caco-2 cells were stimulated with TNFa, the expression of TG2 was completely
blocked in the presence of SB203580, sulfasalazine and Bay-117082 and only
partially by SP600125 (Fig. 3). In contrast, wortmanine, Ly294002, sulfasalazine and
Bay-117082 blocked TG2 induction when Caco-2 cells were stimulated by gIFN.
Therefore, NFkB pathway was central to the induction of TG2 as either TNFa or gIFN
were completely blocked by sulfasalazine and Bay-117082. However, both cytokines
induced different cascades since induction of TG2 by TNFa was selectively blocked by
SB203580 (inhibitor of p38MAPK), while wortmanine (inhibitor of PI3K) and Ly294002
(inhibitor of AKT) selectively blocked the induction of TG2 by gIFN.
Inhibition with sulfosalazine (NFkB) or Ly294002 (AKT) produced a reduction in
TG2 induction in CD tissue samples as well as in control individuals incubated with
TNFa + gIFN (not shown), thus indicating that the same signalling pathways operate
in the small intestine mucosa.
Conclusions
Different proinflammatory cytokines were used to modulate the expression of TG2 in
an in-vitro model of human enterocytes (Caco-2 cells). Among the cytokines tested,
gIFN was the most potent inducer of TG2 expression. A synergistic effect on the expression
of TG2 was observed in cells stimulated with a combination of TNFa+ gIFN.
Similar results were also observed when intestinal biopsies were treated with these
cytokines. Since TNFa and gIFN are present in the small intestine in untreated CD
patients, the higher induction of TG2 by these cytokines must be considered as part of
the pathogenic mechanism in CD.
Specific inhibitors selectively blocked signalling pathways involved in the induction
of TG2 by TNFa and gIFN. These effects were also observed in biopsy samples of the
small intestine mucosa. Since signals producing upregulation of TG2 can participate
in several disease processes and particularly in CD, the knowledge of the molecular
pathways triggering the induction of TG2 constitutes valuable information for the
development of new therapeutic approaches.
References
1. Fesus L, Piacentini M. Transglutaminase 2: an enig matic en zyme with di verse
func tions. Trends Biochem Sci 2002; 27: 534-539.
2. Elli L, Bergamini CM, Bardella MT, et al. Transglutaminases in in flam ma tion
and fi bro sis of the gas tro in tes ti nal tract and the liver. Dig Liver Dis 2009; 41:
541-550.
3. Mehta K, Kumar A, Kim HI. Transglutaminase 2: a multi-task ing pro tein in the
com plex cir cuitry of in flam ma tion and can cer. Biochem Pharmacol 2010; 80:
1921-1929.
4. Caccamo D, Currò M, Ientile R. Po ten tial of transglutaminase 2 as a ther a peu tic
target. Ex pert Opin Ther Tar gets 2010; 14: 989-1003.
5. Schuppan D, Junker Y, Barisani D. Ce liac dis ease: from pathogenesis to novel
therapies. Gastroenterology 2009; 137: 1912-1933.
III. Alter native and novel therapies
Gluten sensitivity: The new kid on the block
of the gluten spectrum disorder
Alessio Fasano
Mucosal Biology Research Center and Center for Celiac Research,
University of Maryland School of Medicine, Baltimore, MD, USA
Wheat, rice, and maize are the most widely consumed food grains in the world.
Wheat, the most widely-grown crop, is immensely diverse, with over 25,000 different
cultivars having been produced by plant breeders worldwide. Much of the world
production of wheat is consumed by humans, after being processed into bread and
other baked goods, pasta and noodles and, in the Middle East and North Africa, bulgar
and couscous. In addition, the broad availability of wheat flour and the functional
properties of gluten proteins provide the rationale for their wide use as an ingredient in
food processing.
Gluten is the main structural protein component of wheat. Possibly, the introduction of
gluten-containing grains, which occurred about 10,000 years ago with the advent of
agriculture, represented a "mistake of evolution" that created the conditions for human
diseases related to gluten exposure, the best known of which are mediated by the
adaptive immune system: wheat allergy and coeliac disease (CD). In both conditions,
the reaction to gluten is mediated by T-cell activation in the gastrointestinal mucosa.
However, in wheat allergy it is the cross-linking of IgE by repeat sequences in gluten
peptides [e.g. Ser-Gln-Gln-Gln-(Gln-)Pro-Pro-Phe] that triggers the release of chemical
mediators, such as histamine, from basophils and mast cells [1]. In contrast, CD,
which affects approximately 1% of the general population, is an autoimmune disorder,
as indicated by specific serologic markers, most notably serum antitissue transglutaminase
(tTG) autoantibodies, by the autoimmune enteropathy that characterizes this
condition, and by autoimmune co-morbidities.
Besides CD and wheat allergy, there are cases of gluten reactions in which neither
allergic nor autoimmune mechanisms are involved. This is generally defined as gluten
sensitivity (GS) [2-5]. Some individuals, who experience distress when eating glutencontaining
products and show improvement when following a gluten-free diet, may
have GS rather than CD. GS patients are unable to tolerate gluten and develop an
adverse reaction when eating gluten that usually, and differently from CD, does not
lead to small intestinal damage. While the gastrointestinal symptoms in GS may
resemble those associated with CD, the overall clinical picture is generally less severe and is not accompanied by the concurrence of tTG autoantibodies or autoimmune
disease. Typically, the diagnosis is made by exclusion, and an elimination diet and "open challenge" (i.e., the monitored reintroduction of gluten-containing foods) are
most often used to evaluate whether health improves with the elimination or reduction
of gluten from the diet.
A number of in-vitro studies have confirmed the cytotoxicity of gluten's main antigen,
gliadin. Gliadin has agglutinating activity, reduces F-actin content, inhibits cell growth,
induces apoptosis, alters redox equilibrium, and causes a rearrangement of the cytoskeleton
through the zonulin pathway and the loss of tight-junction (TJ) competence in
the gastrointestinal mucosa [6-9]. The diversity of gluten-induced conditions is in line
with the notion that the immune system reacts to and deals with the triggering
environmental factor, gliadin, in distinct ways.
The symptoms in GS may resemble those associated with CD [10,11], but with a
prevalence of extra-intestinal symptoms such as ataxia, behavioural changes, bone or
joint pain, muscle cramps, leg numbness, weight loss, chronic fatigue (Table 1).
While the class II MHC haplotype HLA-DQ2 and DQ8 are present in almost all CD
patients, these genes are present in only about 50% of patients with GS, a percentage
slightly higher than in the general population. This suggests a reduced level of
involvement of MHC-dependent, adaptive immune responses in GS relative to CD
[10,11]. In the last decade, several studies have shown that there are signs and
symptoms associated with non-coeliac gluten sensitivity, particularly in the neuropsychiatric
disorders. Persons with schizophrenia have higher than expected titres of
anti-gliadin antibodies, which are related to CD and GS, whereas gluten free diet
seems to improve the behaviour of a subset of children with autism spectrum disorders
(ASDs). However, currently there are no laboratory biomarkers specific for GS.
Usually the diagnosis is done by exclusion, and an elimination diet of glutencontaining
foods, followed by an "open challenge" (monitored reintroduction of
gluten-containing foods). These are most often used to evaluate whether health
improves with the elimination or reduction of gluten from the diet.
Since specific biomarkers need to be developed for specific diagnosis, we propose
that the definition of GS is based on those cases of gluten reaction in which both
allergic and autoimmune mechanisms have been ruled out (diagnosis by exclusion
criteria).
More specifically, these are cases with:
• Neg a tive immuno-al lergy tests to wheat;
• Negative CD serology (EMA and/or tTG) and in which IgA deficiency has been
ruled out;
• Negative duodenal histopathology;
• Possible presence of biomarkers of gluten immune-reaction (AGA+);
• Presence of clinical symptoms that can overlap with CD or wheat allergy
symptoms;
• Resolution of the symptoms following implementation of a GFD.
Based on the aforementioned concept, it is clear that this new form of gluten reaction
within the gluten spectrum disorder needs to be better defined through controlled
studies to define its nature, pathogenesis, proper diagnosis, and management.
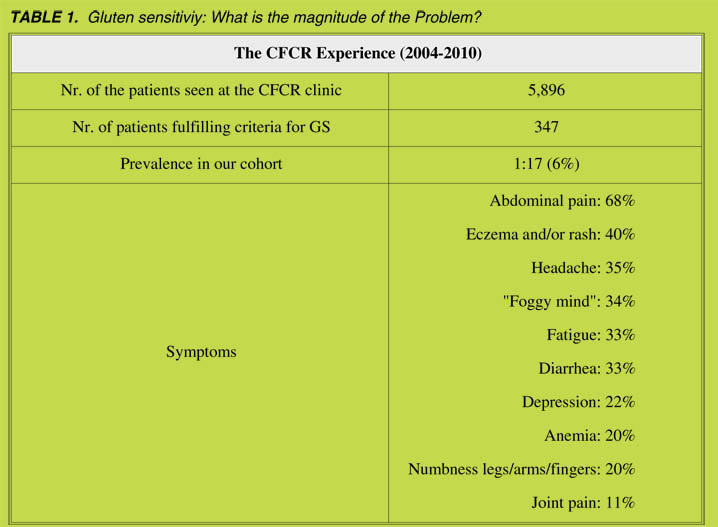
References
1. Fasano A, Catassi C. Cur rent ap proaches to di ag no sis and treat ment of ce liac
dis ease: an evolv ing spec trum. Gastroenterology 2001; 120: 636-651.
2. Presutti RG, Cangemi JR, Cassidy HD. Ce liac dis ease. Am Fam Phys 2007;
76: 1795-1802.
3. Catassi C, Gobellis G. Coeliac dis ease ep i de mi ol ogy is alive and kick ing,
es pe cially in the de vel op ing world. Dig Liver Dis 2007; 39: 908-910.
4. Sood A, Midha V, Sood N, et al. Prev a lence of ce liac dis ease among school
chil dren in Punjab, North In dia. J Gastroenterol Hepatol 2006; 21: 1622-1625.
5. Yachha SK, Poddar U. Ce liac dis ease in In dia. In dian J Gastroenterol 2007;
26: 230-237.
6. Wolters VM, Wijmenga C. Ge netic back ground of ce liac dis ease and its
clinical implications. Am J Gastroenterol 2008; 103: 190-195.
7. Monsuur AJ, de Bakker PI, Alizadeh BZ, et al. My o sin IXB vari ant in creases
the risk of ce liac dis ease and points to ward a pri mary in tes ti nal bar rier de fect.
Nat Genet 2005; 37: 1341-1344.
8. van Bodegraven AA, Curley CR, Hunt KA, et al. Ge netic vari a tion in my o sin
IXB is asso ciated with ulcer ative colitis. Gastroenterology 2006; 131:
1768-1774.
9. Sanchez E, Alizadeh BZ, Valdigem G, et al. MYO9B gene polymorphisms are
as so ci ated with au to im mune dis eases in Span ish pop u la tion. Hum Immunol
2007; 68: 610-615.
10. Sapone A, Lammers K, Mazzarella G, et al. Dif fer en tial mucosal IL-17
ex pres sion in two gliadin-in duced dis or ders: Glu ten sen si tiv ity and the
autoimmune enteropathy celiac disease. Int Arch Al lergy Immunol 2010; 152:
75-80.
11. Sapone A, Lammers MK, Casolaro V, et al. Di ver gence of gut per me abil ity
and mucosal im mune gene ex pres sion in two glu ten as so ci ated con di tions,
celiac disease and gluten sensitivity. BMC Medicine 2011; 9: 23.
Presumptive safety for coeliac patients of
wheat-baked goods rendered gluten-free
during sourdough fermentation
Carlo G. Rizzello, Marco Gobbetti
Dipartimento di Biologia e Chimica Agro-Forestale ed Ambientale,
Università degli Studi di Bari, Bari, Italy
Coeliac disease (CD) is an inheritable disorder of the small intestine that affects
approximately 1% of the world population, and is characterized by an inflammatory
response to ingested wheat gluten and similar proteins in rye and barley, leading to
small intestinal mucosa injury and nutrient malabsorption [1, 2]. The gluten network
is formed by interactions between gliadins (prolamins) and glutenins (glutelins) when
flour and water are mixed. Gluten is unique among the main dietary proteins in that it
contains approximately 15% proline and 35% glutamine aminoacid residues. The
high concentration of glutamine and, especially, praline, prevents proteolysis by
gastric and pancreatic enzymes and results in the building up of oligopeptides, at the
level of the small intestine, which are toxic to CD patients [1].
During the past decades, cereal food technology has changed to such an extent that it has
modified the dietary habits of entire populations that had previously been naïve to
massive gluten exposure. Moreover, baked cereal goods are currently manufactured by
means of fast processes: thus, long-time fermentation through sourdough, a cocktail of
acidifying and proteolytic lactic acid bacteria, has been almost completely replaced by
the use of chemical leavening agends and/or baker's yeast. Cereal components (e.g.,
proteins) are not degraded during production under these technological conditions [1].
CD can be controlled only by maintaining a strictly gluten-free diet (GFD); however,
good compliance is difficult to achieve and some nutritional deficiencies have emerged
[3]. Other therapeutic options, such as the oral supplementation with microbial oligopeptidases,
have been proposed [1].
Recently, several studies were carried out in the author's laboratory, with the aim of
showing the capacity of proteolytic enzymes, mainly peptidases, from selected
sourdough lactobacilli, to degrade gluten during food processing [3-10]. In particular,
several studies showed that pools of lactic acid bacteria (sourdough lactobacilli
and commercial probiotic preparation) under specific processing conditions
(long-time fermentation) had the capacity to markedly hydrolyze and detoxify wheat and rye prolamins fractions, as shown by two-dimensional electrophoresis
and toxicity screening based on agglutination tests on K562(S) cells [4-8]. The
semi-liquid pre-fermentation of wheat flour was another indispensable condition to
fully exploit the potential of enzymes from sourdough lactobacilli [4, 5, 9]. The
processed flour obtained by means of the biotechnological protocol set up in these
pioneer studies was used for manufacturing bread in mixture with containing
gluten-free flours, and for breads used for an in-vivo double-blind acute challenge of
CD patients [5]. Thirteen of the 17 coeliac patients had values of intestinal permeability
that did not differ significantly from the baselines values [5]. The same
approach as those used for wheat flour and bread was adapted for rye and pasta
making, and similar results were obtained [6-8]. In all the cases, although acute
in-vivo and many in-vitro tests were carried out, only a marked decrease in the
gliadin fraction was shown, and further studies were addressed for getting the
complete detoxification of the wheat flour [4-8].
Subsequently, it was shown that a food grade biotechnology consisting of a
combination of 10 selected sourdough lactobacilli with fungal proteases, routinely used
in bakery, decreased the residual concentration of gluten in wheat flour, during food
processing, to below 10 ppm [9]. Fungal proteases are indispensable for generating
polypeptides of intermediate dimensions (e.g., 4-40 amino acids) from native proteins,
which are suddenly transported inside the lactobacilli cells and hydrolyzed through the
complex peptidase system of lactobacilli [10]. It was demonstrated that a large number
of intracellular peptidases (e.g., PepN, PepO, PEP, PepX, PepT, PepV, PepQ and PepR)
are involved in the hydrolysis of the immunogenic polypeptides to free amino acids
[10].
After lactobacilli fermentation, the wheat flour was spray dried. Bread, pasta or sweet
baked goods could be manufactured with the wheat flour rendered gluten-free. Its use
has indubitable economic, nutritional, social and sensory advantages as compared to
its naturally gluten-free counterpart currently used in the GFD. Indeed, as shown by
means of sensory analysis, the properties of baked sourdough goods, manufactured
with wheat flour rendered gluten-free were almost comparable to the full-gluten
counterpart and were highly superior with respect to gluten-free baked goods ([9].
Based on the above results, two independent clinical challenges were carried out,
which involved the daily administration of 200 g of sweet baked goods made with
wheat flour rendered gluten-free (corresponding to ca. 10 g of native gluten) [2, 11].
Eight coeliac patients in remission were enrolled in the first challenge [11].
Even though an intestinal biopsy, showing the typical picture of flat mucosa, is still
considered the gold standard for diagnosing CD, all the routine complementary
analyses (haematology, serology and intestinal permeability analyses) were carried
out in this study [11]. One patient discontinued the trial after 15 days and another after
30 days, mainly due to compliance difficulties during daily consumption. All the other patients showed normal values for haematology, serology and intestinal permeability
during the 60 days of the challenge [11]. In the second trial, 5 patients were challenged
by having to consume baked goods made with processed flour [2]. None of the
patients had clinical complaints over the 60 days; and they did not produce anti-tTG
(anti-transglutaminase) antibodies, nor were modifications of the small intestinal
mucosa visible, and the Marsh grade remained unchanged during the challenge
(Fig. 1) [2].
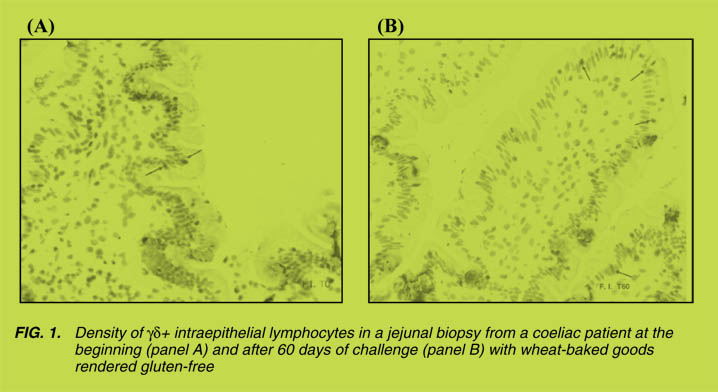
In conclusion we can state that the challenges done demonstrated that a wheat
flour-derived product was not toxic after being administrated over 60 days to CD
patients. Nevertheless, this length of time might not have been sufficiently long to
evaluate toxicity in all coeliac subjects, who may occasionally show a different
sensitivity to gluten [2, 11].
New and longer trials have been planned to demonstrate the safety of baked goods
manufactured by applying this promising and recently rediscovered biotechnology.
References
1. Gobbetti M, De Angelis M, Di Cagno P, et al. Sour dough lactobacilli and
celiac disease. Food Microbiol 2007; 24: 187-196.
2. Greco L, Gobbetti M, Auricchio R, et al. Safety for pa tients with ce liac dis ease
of baked goods made of wheat flour hy dro lyzed dur ing food pro cess ing.
Clin Gastroenterol Hepatol 2011; 9(1): 24-29.
3. Di Cagno P, Rizzello CG, Gagliardi F, et al. Differ ent fecal microbiotas and
vol a tile or ganic com pounds in treated and un treated chil dren with ce liac
disease. Appl Environm Microbiol 2009; 75: 3963-3971.
4. Di Cagno R, De Angelis M, Lavermicocca P, et al. Pro te ol y sis by sour dough
lac tic acid bac te ria: ef fects on wheat flour pro tein frac tions and gliadin pep tides
in volved in hu man ce real in tol er ance. Appl Environm Microbiol 2002; 68:
623-633.
5. Di Cagno R, De Angelis M, Auricchio S, et al. Sour dough bread made from
wheat and non toxic flours and started with se lected lactobacilli is tol er ated in
ce liac sprue pa tients. Appl Environm Microbiol 2004; 70: 1088-1096.
6. Di Cagno P, De Angelis M, Alfonsi G, et al. Pasta made from durum wheat
sem o lina fer mented with se lected lactobacilli as a tool for a po ten tial de crease
of the glu ten in tol er ance. J Agric Food Chem 2005; 53: 4393-4402.
7. De Angelis M, Rizzello CG, Fasano A, et al. VSL#3 probiotic prep a ra tion has
the ca pac ity to hy dro lyze gliadin polypeptides re spon si ble for ce liac sprue.
Bioch Biophys Acta 2006; 1762: 80-93.
8. De Angelis M, Coda R, Silano M, et al. Fer men ta tion by se lected sour dough
lac tic acid bac te ria to de crease coeliac in tol er ance to rye flours. J Ce real Sci
2006; 43: 301-314.
9. Rizzello CG, De Angelis M, Di Cagno R, et al. Highly ef fi cient glu ten
deg ra da tion by lactobacilli and fun gal pro teas es dur ing food pro cess ing:
new per spec tives for ce liac dis ease. Appl Environm Microbiol 2007; 73:
4499-4507.
10. De Angelis M, Cassone A, Rizzello CG, et al. Mech a nism of deg ra da tion of
immunogenic glu ten epitopes from Triticum turgidum L. var. durum by
sour dough lactobacilli and fun gal pro teas es. Appl Environm Microbiol 2011;
76: 508-518.
11. Di Cagno P, Barbato M, Di Camillo C, et al. Glu ten-free sour dough wheat
baked goods ap pear safe for young ce liac pa tients: a pi lot study.
J Pediatr Gastroenterol Nutr 2010: 51: 777-783.
Transamidation of wheat: An enzyme strategy to detoxify gluten
Paolo Bergamo1, Carmen Gianfrani1, Federica Capobianco2, Salvatore
Moscaritolo 2, Mauro Rossi 1
1 Institute of Food Sciences, National Research Council, Avellino, It aly
2 IPALC Research & Development Laboratories, Frigento-AV, Italy
Introduction
Coeliac disease (CD) is characterized by the activation of intestinal gluten-specific
CD4+ T cells. In particular, gluten becomes a better T cell antigen following deamidation
catalyzed by tissue transglutaminase (tTG) [1]. We reported that a preventive
transamidation of gliadin by a single incubation of wheat flour with microbial TG
(mTG) and lysine methyl ester (K-CH3), completely inhibited the IFN-g expression of
intestinal gliadin-specific T cell lines from CD patients [2].
More recently, we showed that a protracted intake of this transamidated gluten was
tolerated in a subset of CD patients [3].
The present work investigates the effects on the adaptive immune response in vitro
and the technological properties of transamidated wheat flour and semolina.
Methods
A wheat flour suspension, depleted of the albumin/globulin fraction was subjected to
two sequential steps of transamidation with mTG (ACTIVA®WM; 81-135 U/g;
Ajinomoto Foods Hamburg, Germany) and lysine ethyl ester (K-C2H5; NutraBio.com,
Middlesex, NJ, USA) at 30 °C. Soluble proteins and gliadin fractions were recovered
and analyzed by SDS-PAGE.
Intestinal T-cell lines were isolated from three HLA-DQ2+ adult CD patients and incubated
with various preparations of pepsin/trypsin (pt)-digested and tTG-deamidated
gliadin. Culture supernatants were analyzed for IFN-g protein secretion by ELISA.
Bread wheat flour or durum wheat semolina was suspended in water containing 8 U/g
mTG and 20 mM K-C2H5. Incubation was performed in two steps: first step, 2 h at 30 °C
followed by centrifugation (1100 g 10 min); second step, 3 h at 30 °C with fresh enzyme
and K-C2H5. The suspension was finally centrifuged to recover dough. A bread baking
procedure was tested with 500 g of dough mixed with 3.0 g table salt and 10.0 g baker’s yeast. A pasta making machine was used to make pasta (spaghetti) with a length of
200 mm and a thickness of 1.75 mm. The pasta was dried according to the following
schedule: 90 °C, 83% relative humidity (rh) 3 h; 63 °C, 73 % rh 1 h; 40 °C, 70% rh 90
min, room temperature 48 h. The prolamin content in transamidated products was
determined by means of R5 ELISA (Istituto Ricerche Agrindustria, Modena, Italy).
Results
The transamidation reaction generated a soluble form of gliadin along with
transamidated insoluble gliadin (Fig. 1A). Notably, we confirmed that transamidated
insoluble gliadin was inactive, whereas soluble gliadin was still active when
challenged in vitro with intestinal T-cell lines from CD patients (Fig. 1B).
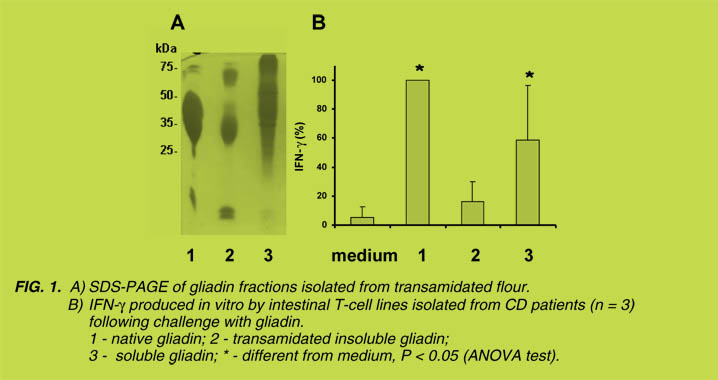
We found that the gluten content in transamidated bread, determined by means of R5
ELISA, drastically decreased from 1102.7 mg/kg, after the single step, to 5.8 mg/kg
after the two-step reaction (Table 1). A similar result was obtained for transamidated
semolina (Table 1), suggesting that prolamins were extensively masked following a
two-step transamidation process. The transamidated wheat bread had a wheat-like
flavour, a brown crust colour and a crumb structure similar to the control bread (Fig.
2A). Our findings, however, showed that the specific volume was lower than in the
control bread (2.21 vs. 2.69 ml/g, transamidated vs. control). Similarly, a dried pasta
was produced with transamidated semolina (Fig. 2B). The water uptake of transamidated
pasta following cooking was comparable to that of untreated pasta (146%
vs. 149%; transamidated vs. control).
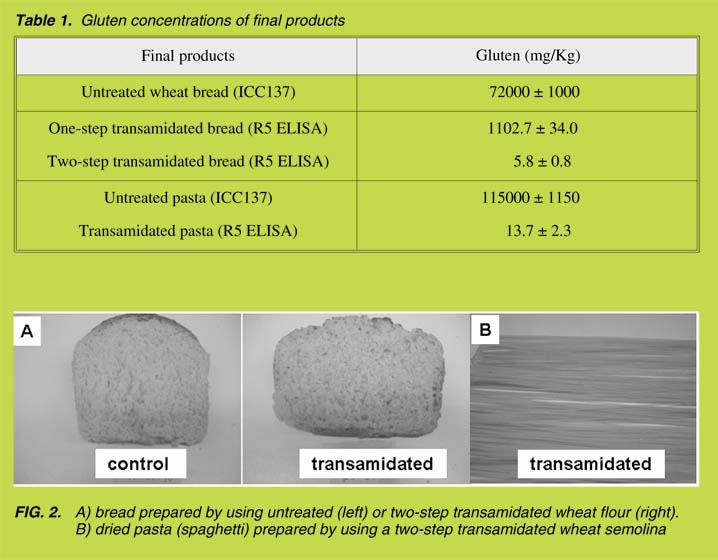
Conclusions
This study shows that transamidation generated a soluble form of gliadin that continued
to be immunologically active, along with inactive insoluble gliadin. The two-step
transamidation processing of wheat flour or semolina eliminated this fraction and
completely blocked the immune recognition of wheat prolamins by R5 monoclonal
antibody. This treatment did not hamper the main technological properties of gluten, as
good quality bread and dried pasta were produced. The in-vivo safety of the two-step
transamidated wheat flour for coeliac patients is under investigation.
Aknowledgements
This study was supported by grants from the CNR (Annualità 2008), IPAFOOD srl,
Ente Provincia Avellino (Annualità 2009) and Associazione Italiana Celiachia sez.
Campania. We thank Ajinomoto Foods Europe SAS for the generous supply of
ACTIVA®WM.
References
1. Molberg O, McAdam SN, Korner R, et al. Tissue transglutaminase selectively
mod i fies gliadin pep tides that are rec og nized by gut-de rived T cells in ce liac
disease. Nat Med 1998; 4: 713-717.
2. Gianfrani C, Siciliano RA, Facchiano AM, et al. Transamidation in hib its the
in tes ti nal im mune re sponse to gliadin in vitro. Gastroenterology 2007; 133:
780–789.
3. Mazzarella G, Salvati VM, Iaquinto G, et al. Transamidation of wheat flour:
a new en zyme strat egy to block glu ten tox ic ity in a sub set of ce liac dis ease
pa tients. GAS TRO ’09, Lon don, UK. Gut 2009; 58 (Suppl II): A80.
Peptidases for degradation of gluten and possible use in dietary therapy
Pe ter Koehler, Her bert Wieser
German Re search for Food Chemistry, Freising, Germany
Introduction
Coeliac disease (CD) is a common inflammatory disease of the small intestine that is
triggered by the storage proteins of wheat, rye, barley and, possibly, oats in geneticallypredisposed
individuals. This group of proteins (wheat gliadins and glutenins, rye
secalins, barley hordeins, oat avenins) is collectively referred to as ‘gluten’. The current
treatment for CD is a strict, lifelong, gluten-free diet to prevent chronic enteropathy and
reduce the risk of lymphoma and carcinoma. The maximum daily intake of gluten
should not exceed 20 mg, which corresponds to around one hundredth of a slice of
bread. This dietary restriction, however, is a big challenge for CD patients and may lead
to poor compliance or inadvertent intake of gluten. There is, therefore, an urgent need to
develop safe and effective therapeutic alternatives and, based on the advanced
understanding of the pathomechanism of CD, non-dietary therapies have been devised
during the last decade. Examples include modified flours that have been depleted of
immuno-dominant gluten epitopes, or a decrease in intestinal permeability by blocking
the epithelial zonulin receptor, inhibition of intestinal transglutaminase activity,
inhibition of proinflammatory cytokines and inhibition of peptide presentation by
HLA-DQ2 antagonists (recently reviewed by Schuppan et al. [1]). Intense clinical trials
will be necessary to ensure the effectivity of such alternatives.
Peptidases for gluten degradation
The structural features unique to all CD-toxic proteins are amino acid sequence
domains rich in glutamine (Q) and proline (P) [2]. The high proline content,
particularly in the repetitive sequence motifs, renders these proteins resistant to
complete proteolytic digestion by human gastrointestinal enzymes. Consequently,
large proline- and glutamine-rich peptides are accumulated in the small intestine;
a striking example is the ‘33-mer’ peptide from a-gliadins (sequence:
LQLQPFPQPQLPYPQPQLPYPQPQLPYPQPQPF), which has been shown to be
resistant to gastric and pancreatic peptidase-mediated digestion [3]. Such peptides
penetrate into the subepithelial lymphatic tissue, and, depending on the amino acid
sequences, induce two different immune responses: the rapid innate response and the slower adaptive response [1, 2]. Hence, one strategy to prevent those peptides
from reaching the lymphatic tissue has been to make use of special peptidases that
cleave large toxic peptidases into small non-toxic fragments with less than nine
amino acid residues (‘oral enzyme therapy’). In addition, peptidases have been
recommended for decreasing the level of gluten proteins and peptides in raw
material for food production (e.g. starch) or food (e.g. beer) before ingestion by CD
patients (‘detoxification of gluten’). Bacteria, fungi and germinating cereals have
been proposed as sources for gluten-degrading peptidases.
Koshla’s and Sollid’s groups were the first to make use of bacterial peptidases (prolyl
endopeptidases, PEPs) for the detoxification of gluten peptides [3, 4]. They are
expressed in various microorganisms such as Flavobacterium meningosepticum,
Sphingomonas capsulata, and Myxococcus xanthus [5]. The effectiveness of these
PEPs, however, were limited by restrictions on the length of their substrates, their sensitivity
against low pH (< 5) and pepsin, and the long duration necessary to completely
digest gluten peptides [1]. However, PEPs from Sphingomonas capsulata, in combination
with endopeptidase B2, a glutamine-specific peptidase from barley, has been
shown to have an effective synergistic potential for the detoxification of gluten proteins
and peptides [6, 7]. The combined enzymes are currently being tested in phase 2a
clinical studies to evaluate safety and efficacy.
Koning’s group studied a prolyl endopeptidase from Aspergillus niger (AN-PEP),
which is suitable for both the removal of gluten from food as well as from oral supply
[8, 9]. Its advantages are, in comparison to bacterial PEPs, the resistance to low pH
and to pepsin activity, degradation of both proteins and peptides, and, in addition,
strains of the genus Aspergillus have a food-grade status. It was demonstrated that
AN-PEP degraded gluten to non-toxic fragments by digesting a food matrix in an in
vitro gastrointestinal system [9, 10]. Phase 1 clinical trials did not indicate any toxicity
of the enzyme; and phase 2 trials are in preparation.
Sourdough lactobacilli have been shown to produce specific peptidases that
hydrolyse proline-rich peptides [11]. Together with fungal peptidases they are
capable to degrading gluten in sourdough so that treated sourdough can be used as a
gluten-free ingredient in gluten-free formulations for baked goods.
Cereal peptidases have long been known to degrade storage (gluten) proteins during the
germination of seed to provide the embryo with amino acids. Therefore, they were
tested for their capability to detoxify gluten peptides by means of extensive fragmentation
[12]. A highly active peptidase fraction extracted from germinated rye bran has
been shown to cleave CD-toxic peptides into fragments with less than nine amino acids
very quickly and at different pH values (Fig. 1). Moreover, they are able to degrade
intact gluten proteins. Peptidase preparations from germinated cereals have distinct
advantages as compared to PEPs from microorganisms. They contain mixtures of endoand
exopeptidases with optimal specificities for gluten degradation. The enzymes derive from a naturally safe food source and no genetic engineering is necessary. Their
production is part of a well-established technological process (malting, beer production)
and is rather simple and cheap. Therefore, peptidase preparations from germinated
cereals are promising candidates for the detoxification of gluten-containing foods, as
was recently shown by the application on beverages such as kwas and malt beer [13],
and, possibly, for the oral therapy of CD patients.
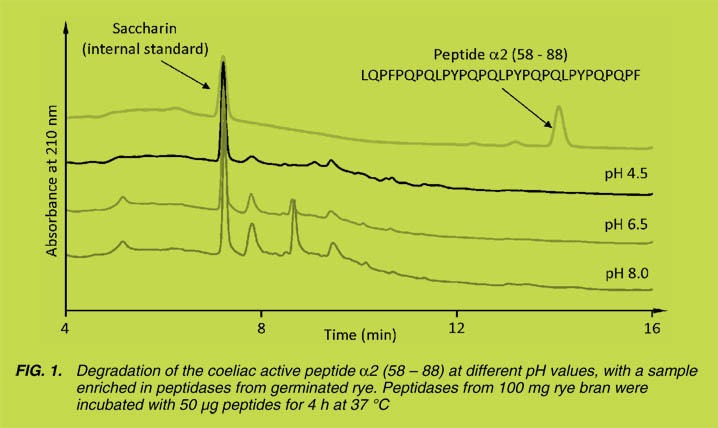
Conclusion
A series of studies has been conducted on the preparation and application of peptidases
capable of degrading gluten and gluten-derived peptides into fragments non- toxic to
CD patients. Peptidase sources are bacteria, fungi and germinated cereals. Potential
applications can be divided into treatment of gluten-containing food as well as therapeutic
use. In principle, gluten-containing raw material and food can be effectively
rendered gluten-free by peptidase treatment, which is advantageous in products that do
not need gluten functionality for quality parameters. In terms of oral therapy, it should
be pointed ou that most CD patients can be cured effectively by means of a gluten-free
diet, which has no potential side-effects. Alternative therapies should have a similar
safety profile and one must carefully weigh the risks, benefits, and the costs of
alternatives. In particular, the issue about whether peptidase samples for oral use should
be applied on a regular or occasional basis still needs to be discusssed in depth.
References
1. Schuppan D, Junker Y, Barisani D. Ce liac dis ease: from pathogenesis to novel
therapies. Gastroenterology 2009; 137: 1912-1933.
2. Wieser H, Koehler P. The bio chem i cal ba sis of ce liac dis ease. Cereal Chem
2008; 85: 1-13.
3. Shan L, Molberg Ø, Par rot I, et al. Struc tural ba sis for glu ten in tol er ance in
celiac sprue. Sci ence 2002; 297: 2275-2279.
4. Hausch F, Gray G, Shan L, et al. Enzyme treatment of foodstuffs for ce liac
sprue. 2003. Patent No. WO 2003/068170.
5. Shan L, Marti T, Sollid L, et al. Compar ative biochem ical analysis of
three bac te rial prolyl endopeptidases: im pli ca tions for coeliac sprue.
Biochem J 2004; 383: 311-318.
6. Siegel M, Bethune MT, Gass J, et al. Ra tio nal de sign of com bi na tion en zyme
ther apy for ce liac sprue. Chem Biol 2006; 13: 649-658.
7. Gass J, Bethune MT, Siegel M, et al. Com bi na tion en zyme ther apy for gas tric
di ges tion of di etary glu ten in pa tients with ce liac sprue. Gastroenterology
2007; 133: 472-480.
8. Stepniak D, Spaenij-Dekking L, Mitea C, et al. Highly efficient glu ten
deg ra da tion with a newly iden ti fied prolyl endoprotease: im pli ca tions for ce liac
disease. Am J Physiol 2006; 291: G621-G629.
9. Edens L, van der Hoeven RAM, de Roos AL, et al. Use of proline-spe cific
endoproteases to hydrolyse proline-rich peptides at acid pH in food pro cess ing.
2005. Patent No. WO 2005/027953.
10. Mitea C, Haavenaar R, Drijfhout J, et al. Ef fi cient deg ra da tion of glu ten by a
prolyl endoprotease in a gas tro in tes ti nal model: im pli ca tions for coeliac
disease. Gut 2008; 57: 25-32.
11. Gobetti M, Rizello CG, Di Cagno R, et al. Sour dough lactobacilli and ce liac
disease. Food Microbiol 2007; 24: 187-196.
12. Hartmann G., Koehler P., Wieser H. Rapid deg ra da tion of gliadin pep tides
toxic for coeliac dis ease pa tients by pro teas es from ger mi nat ing ce re als.
Cereal Sci 2006; 44: 368-371.
13. Koehler P, Geßendorfer B, Wieser H. Preparation of partially hy dro lysed
prolamins as references for the immunochemical quantification of gluten in
ce real-based beverages. In: Stern M, ed. Proceedings of the 23rd Meeting of
the Working Group on Prolamin Analysis and Toxicity. September 25-27,
2008, Barcelona, Spain. Zwickau: Verlag Wissenschaftliche Scripten 2010;
35-40.
IV. AO ECS
AOECS Association of European Coeliac Societies*
Hertha Deutsch
AOECS-Codex-Delegate, Vienna, Austria
Introduction
AOECS is the umbrella organisation of national coeliac societies in Europe and is an
independent non-profit association. AOECS was founded in 1988 and comprises today
37 coeliac societies from the following 33 countries:
Andorra, Austria, Belgium (2), Bosnia-Herzegovina, Bulgaria, Croatia, Cyprus, Czech
Republic (2), Denmark, Estonia, Finland, France, Germany, Greece (2), Hungary, Ireland,
Italy, Luxembourg, Malta, Montenegro, Netherlands, Norway, Poland, Portugal,
Russia/St.Petersburg, Serbia, Slovenia, Slovakia, Spain (2), Sweden, Switzerland,
Ukrainia and the United Kingdom.
AOECS works on subjects of international importance regarding coeliac disease and
the gluten-free diet, coordinates international activities and matters of common interest
of the members, exchanges information among the members, gives any possible advice
and assistance to small and recently formed coeliac societies and is lobbying for
awareness of the gluten intolerance.
Gluten-free diet – legal improvements
Since 1992 AOECS has the status "Observer" in the world-wide Codex Alimentarius
Commission and participated in some Codex Committees and all sessions of the
Commission since that time. In various AOECS statements the coeliac disease, the
need for correct labelling of foods and the high incidence of the gluten intolerant
population was brought to the attention of the governments and food manufacturing
companies all over the world. This resulted in the fact, that the awareness of gluten
intolerance increased considerably in the general food industry worldwide.
AOECS participated very actively in the elaboration and modification of all worldwide Codex Standards and Guidelines for labelling of foods for normal consumption,
genetically modified foods and special dietary foods. Further details of all relevant
texts and references were written in the paper "20 years AOECS" published in the Proceedings of the 23rd Meeting of the Working Group on Prolamin Analysis and
Toxicity from 25-27 September 2008. As usual Codex Standards are transferred into
national food legislation and these improvements have been implemented in national
food legislation e.g. EU-Directives.
With the adoption of the "Codex Standard for Foods for Special Dietary Use for Persons
Intolerant to Gluten" in July 2008 and the equivalent "Commission Regulation (EC) No
41/2009" of January 2009 all legal requirements for a save gluten-free diet were
established and the most important aim for coeliacs fulfilled.
International Gluten-free Symbol

The "Crossed-Grain-Symbol", known as the "Gluten-free Symbol" has been used on
special dietary products and information material about coeliac disease since more
than 40 years. The owner of this symbol is Coeliac UK who gave permission for usage
to national coeliac societies. Because of different national interpretations about the
term "gluten-free" the meaning of the Gluten-free Symbol was very varying within
Europe. The AOECS Working Group Codex, Labelling and Symbol elaborated the "European Licensing-System" which was adopted by the AOECS General Assembly
in September 2009.
European Licensing System
The benefit of the European Licensing System is the harmonisation for the usage of
the Crossed-Grain-Symbol in all countries within and outside Europe. It consists of
the AOECS Standard, the AOECS Charta and the Licensing Contract.
AOECS Standard
The "AOECS Standard for Foods for Persons Intolerant to Gluten" is based on the
worldwide "Codex Standard for Foods for Special Dietary Use for Persons Intolerant
to Gluten" and is an integral part of the License Contract. It describes the food groups
which are permitted bearing the Symbol, specifies technical requirements, determines the thresholds, the analytical method, the frequency of analytical testing and has six
Annexes:
An nex I: List of food prod ucts which are not per mit ted bear ing the Crossed
Grain Sym bol be cause these prod ucts are al ways glu ten-free e.g. fruits,
milk etc.
Annex II: HACCP Guidance for flour, flour mixture and mill
Annex III: HACCP Guidance for bakery prod ucts
Annex IV: HACCP Guidance for pasta pro duc tion
Annex V: HACCP Guidance for foods for general con sump tion
Annex VI: HACCP Guidance for confectionary products.
AOECS Charta
The AOECS Charta is the contract between AOECS and its member associations to
guarantee a unified European Licensing System of the Crossed Grain Symbol on food
products in the AOECS Territory. The Charta regulates all necessary procedures e.g.
certification guidance, license fees, administration, monitoring and the registration
numbering system.
License Contract
The License Contract is the agreement between the food producer and the national
coeliac society. It guarantees that coeliacs can rely that products bearing the Crossed-
Grain-Symbol with the registration number below fulfil the same technical requirements
in all countries in terms of avoiding contamination controlled by the same
analytical testing which is for the time being the R5-method.
Development in CCMAS
In March 2010 the day before the plenary session of CCMAS (Codex Committee on
Methods of Analysis and Sampling) a workshop was organised by IAM/MoniQA
where the item "Proprietary Methods" was discussed among other subjects. Although "Proprietary Methods" is a general issue, special attention was given to the R5-method.
A lot of concerns were raised by some delegates, e.g.: regarding availability of reagents;
restricted licensing of antibodies; the danger that the adoption of a proprietary method
created an anti-competitive situation; methods should have an independent validation,
not manufacturer's validation; to describe the method more generically for the purpose
of use as a Codex method etc.
AOECS explained the difference between the Skerritt and the R5-method and draw
the attention of the delegates to the result if different methods will be adopted by
Codex: we know from several studies that the same food sample tested with different
antibodies, standards and methods give extremely varying results. The consequence
for the European Alert System and food labelling legislation has to be considered if
nobody knows which test is right and which is wrong. AOECS strongly recommended
that an independent scientist or small group of scientists, who have outstanding
knowledge about gluten analysis, should make a ring test comparing well known and
new methods aiming to suggest the best method from the scientific point of view.
Further on AOECS recalled that because of the request of Codex the Prolamin Working
Group was established more than 20 years ago to advise Codex regarding gluten
analysis. Few years ago the Prolamin Working Group recommended the R5 method.
At the plenary session of CCMAS at the agenda Nr. 8 "Report of an interacengy
meeting on methods on analysis and sampling" this item was discussed. The IAM had
prepared a first draft paper, presented in the Annex to CRD 2 for information. The
paper noted that proprietary methods were not clearly defined, highlighted some
concerns that could arise from their use: they might prevent further development of
new and better techniques, distort competition between companies producing the
reagents, and create difficulties for government authorities if particular reagents were
not readily available for official methods. It was recalled that the R5 method for the
determination of gluten illustrated some of these problems as the reagents were not
generally available. Several approaches were proposed in CRD 2 to address this issue,
including the use of the criteria approach in Codex.
The Delegation of New Zealand recalled that it had earlier proposed to put forward a
new procedure for evaluation of methods, and offered to contribute to future discussions
on this issue.
AOECS contributes in the discussion. The statement is included in the CCMAS report
as follows:
"The Observer from AOECS recalled that the R5 method is the most accurate method
from the scientific point of view for the time being, and that if different methods were
allowed, it would create serious problems as to how to handle different results for the
same food sample: if one method detects a gluten content higher than 20 mg/kg gluten
and another method detects a level below 20 mg/kg, it cannot be determined whether
the food can be labelled "gluten free" or not. Moreover, the Observer noted that a
method which underestimates the gluten content in foods poses severe health risks for
gluten intolerant-consumers."
It was noted that the IAM would proceed with its consideration of proprietary
methods, invited wider contribution than only IAM members and would provide an
update to the next session of the Committee.
AOECS kindly asks the Prolamin Working Group to consider this new development in
CCMAS and asks very urgent to consider whether the Prolamin Working Group or a
group member could take over the very important work to compare all existing gluten
analysis to give the best possible advise to Codex in this extremely important issue for
the consumers health, the food industry and national food authorities.
V. Perspectives and action plan
Perspectives and action plan
Mar tin Stern
University Children's Hospital, Tuebingen, Germany
The Prolamin Working Group executive meeting and joint discussion held on October 1,
2010, led to the decisions outlined below.
Action plan
I. Analytical
• PWG gliadin is avail able in 100 mg batches from the PWG chair man
(mar tin.stern@med.uni-tuebingen.de).
• R5 ELISA for glu ten anal y sis based on PWG gliadin con tin ues to be the ac cepted
ba sis for glu ten anal y sis. New test sys tems will have to be tested by ring trial.
II. Clinical
• Non-coeliac glu ten sen si tiv ity will be in cluded in the spec trum of group ac tiv i ties.
• The rel e vance of glu ten und mod i fied glu ten in food tech nol ogy will be a fur ther
focus.
III. Publication and policy
• The PWG home page (http://www.wgpat.com.ar) will be ex tended to in clude
pho to graphs and re search pro files of group mem bers as well as ta ble of con tents
of the most re cent meet ing.
• New group mem bers from sci ence and in dus try will be searched ac tively.
• The programme for the group meet ing in Stuttgart-Fellbach 2011 will be pub lished
al ready in April 2011 (printed flyer in col lab o ra tion with Deut sche Zöliakie-
Gesellschaft e. V. (DZG).
• This printed, citable book ver sion of the 2010 pro to col (print run: 500 cop ies, with
ISBN) was made pos si ble through fund ing by Dr. SCHÄR GmbH/Srl, Burgstall
BZ, It aly), and by the ef forts of the pro duc tion team of Verlag Wissenschaftliche
Scripten, Au er bach, Ger many. It will be dis trib uted among lead ers of opin ion in
glu ten analy sis and clinical med icine.
|
|
List of Participants
GROUP MEMBERS
Prof. Dr. Carlo Catassi
Università politecnica delle marche Facoltà di Medicina e Chirurgia
Istituto di Clinica Pediatrica
Via Corridoni 11 - 60123 ANCONA, IT ALY
Phone +39 349 2235 447 - Tele fax +39 071 36281
Email: catassi@tin.it
Prof. Dr. Fernando G. Chirdo
Laboratorio de Investigación en el Sistema Inmune (LISIN)
Facultad de Ciencias Exactas Universidad Nacional de La Plata cc 711
(1900) LA PLATA, AR GEN TINA
Phone +54 221 421 0 497, 423 0 121, 423 5 333 (Int 45)
Tele fax +54 221 422 6947
Email: fchirdo@biol.unlp.edu.ar
Prof. Paul J. Ciclitira
King's Col lege Lon don (Di vi sion of Di a be tes and Nu tritional Sciences) The Rayne In sti tute (KCL). St Thomas' Hos pi tal Westminster Bridge Road. LON DON SE1 7EH, UK/ENG LAND
Phone +44 207 620 2597; 207 188 2494 - Telefax +44 207 261 0667
Email: mila.labar_weintrop@kcl.ac.uk (secretary)
Email: paul.ciclitira@kcl.ac.uk
Prof. Dr. Pe ter Köhler
Deut sche Forschungsanstalt für Lebensmittelchemie
Lise-Meitner-Straße 34 - 85354 FREISING, GER MANY
Phone +49 81 61 71 29 28 - Tele fax +49 81 61 71 29 70
Email: pe ter.koehler@tum.de
Prof. Dr. Frits Koning
Leiden Univer sity Medical Center, E3-Q
De part ment of Immunohaematology and Bloodbank Albinusdreef 2
2333 ZA LEIDEN, THE NETHERLANDS
Phone +31 71 5266673 - Tele fax +31 71 5265267
Email: fkoning@lumc.nl
Prof. Dr. Thomas Mothes
Universitätsklinikum Leip zig A. ö. R.
Institut für Laboratoriumsmedizin, Klinische Chemie und Molekulare Diagnostik
Liebigstraße 27 - 04103 LEIP ZIG, GER MANY
Phone +49 341 97 22251 - Tele fax +49 341 97 22329
Email: mothes@medizin.uni-leip zig.de
Prof. Dr. Mar tin Stern
Universitätsklinik für Kinder- und Jugendmedizin Hoppe-Seyler-Straße 1
72076 TÜBINGEN, GER MANY
Phone +49 7071 29 83781 -
Tele fax +49 7071 29 5477
Email:
mar tin.stern@med.uni-tuebingen.de
Prof. Conleth Feighery, MD
(not at tend ing)
Uni ver sity of Dub lin De part ment of Im mu nol ogy St. James's Hos pi tal
James's Street DUB LIN 8, IRE LAND
Phone +353 1 896 3432 - Tele fax +353 1 4545-609
Email: con.feighery@tcd.ie
Dr. Frederik W. Janssen
(not at tend ing)
Fascoda Food Con sul tancy Coehoornsingel 44
7201 AD ZUTPHEN, THE NETHERLANDS
Phone +31 575 515948 - Tele fax +31 575 512207
Email: fwjanssen@chello.nl
Katri Kaukinen, MD, PhD
(not at tend ing)
Uni ver sity of Tampere Medical School, Paediatric Research Centre
De part ment of Gastroenterology and Al i men tary
Building Finn-Medi 3
33014 UNIVERSITY OF TAMPERE, FINLAND
Phone +358 3 3551 8403 - Telefax: +358 3 3551 8402
Email: katri.kaukinen@uta.fi
Prof. Riccardo Troncone
De part ment of Pe di at rics and Eu ro pean Lab o ra tory for the In ves ti ga tion of Food-in duced Dis eases. Uni ver sity of Na ples "Federico II"
via Pansini, 5 - 80131 NA PLES, IT ALY
Phone +39 081 7463383 - Tele fax +39 081 5469811
Email: troncone@unina.it
Dr. Renate van Eckert
Victo ria Univer sity of Wellington
Centre of Biodiscovery and School of Biological Sciences
P.O. Box 600 WELLINGTON, NEW ZEA LAND
Phone +64 4 463 6092 - Tele fax +64 4 463 5331
Email: renate.vaneckert@gmail.com
HOST
Prof. Dr. Carlo Catassi
Università politecnica delle marche Facoltà di Medicina e Chirurgia
Istituto di Clinica Pediatrica
via Corridoni 11 - 60123 ANCONA, IT ALY
Phone +39 349 2235 447 - Tele fax +39 071 36281
Email: catassi@tin.it
Elisa Giordano
Pro ject Assistant Attività Residenziali Fo rum Ser vice srl
Part ner organizzativo di Accademia Nazionale di Medicina
Via M. Piaggio 17/6 - 16122 GENOVA, IT ALY
Phone +39 01083794233 - Telefax +39 1083794261
Email: giordano@accmed.org
GUESTS
Tova Almlöf
Semper AB Löfströms Allé 5
172 22 SUNDBYBERG, SWE DEN
Phone +46 8 505 93 134 - Tele fax +46 8 505 93 156
Email: tova.almlof@sem per.se
Sofia Beisel
Deut sche Zöliakie-Gesellschaft e.V. Kupferstraße 36
70565 STUTTGART, GER MANY
Phone +49 711 459981 15 - Tele fax +49 711 459981 50
Email: sofia.beisel@dzg-on line.de
Dr. Helen Brown
Biochemistry Section Manager Campden BRI
CHIP PING CAMPDEN,GLOS. GL55 6LD, UK
Phone +44 1386 842016 - Tele fax +44 1386 842100
Email: h.brown@campden.co.uk
Dr. Virna Cerne
Schär R&D Cen tre - Dr. SCHÄR GmbH c/o Area Sci ence Park
Padriciano 99. 34012 TRIESTE, IT ALY
Phone +39 040 3755383 - Tele fax +39 040 3755385
Email: virna.cerne@schaer.com
Josè D'Alessandro
Istituto di Scienze dell'Alimentazione
Consiglio Nazionale delle Ricerche
Via Roma 64. 83100 AVELLINO, IT ALY
Phone +39 0825 299 391 - Tele fax +39 0825 299 104
Email: jallesandro@isa.cnr.it
Dr. Sigrid Haas-Lauterbach
R-Biopharm AG An der neuen Bergstraße 17
64297 DARMSTADT, GER MANY
Phone +49 6151 81 0225 - Tele fax +49 6151 81 02 20
Email: s.h.lauterbach@r-biopharm.de
Stefania Iametti, PhD
Research Professor Biochemistry. Università degli studi di Milano
Dipartimento di Scienze Molecolari Agroalimentari (DISMA)
Via Celoria, 2. 20133 MI LANO, IT ALY
Phone +39 02 5031 6800 - Tele fax +39 02 5031 6801
Email: stefania.iametti@unimi.it
Dr. Ulrike Immer
R-Biopharm AG
An der neuen Bergstraße 17. 64297 DARMSTADT, GER MANY
Phone +49 6151 6802 38 - Tele fax +49 6151 6802 20
Email: u.immer@r-biopharm.de
Päivi Kanerva
De part ment of Food and Evironmental Sciences
P.O.Box 66 Agnes Sjöbergin katu 2
00014 UNI VER SITY OF HEL SINKI, FIN LAND
Phone +358 9 191 58236 - Tele fax +358 9 191 58460
Email: paivi.kanerva@hel sinki.fi
Giuseppe Mazzarella
Istituto di Scienze dell'Alimentazione
Consiglio Nazionale delle Ricerche
Via Roma 52. 83100 AVELLINO, IT ALY
Phone +39 0825 299 391 - Tele fax +39 0825 299 104
Email: gmazzarella@isa.cnr.it
Norma McGough
Coeliac UK, 3rd Floor, Apollo Cen tre Desborough Road
HIGH WYCOMBE
BUCKINGHAMSHIRE HP11 2QW. UK/ENG LAND
Phone +44 1494 796135 - Tele fax +44 1494 474349
Email: norma.mcgough@coeliac.org.uk
Jonatan Miranda Gomez
Uni ver sity of the Basque Coun try Dept. Nu tri tion & Food Sci ences
Paseo de la Universidad, 7. 01006 VITORIA-GASTEIZ, SPAIN
Phone +34 94 501 3863
Email: jonatan-miranda@ehu.es
Susanna Neuhold
Coordinatore Area Alimenti Food Area
Man ager Italian Coeliac Association
Via Caffaro 68 A rosso. 16124 GENOVA, IT ALY
Phone +39 010 2510235 - Tele fax +39 010 2721615
Email: sneuhold@celiachia.it
Email: alimenti@celiachia.it - segretaria@celiachia.it
Dr. Juan Ignacio Serrano Vela
Asociación de Celíacos de Ma drid (ACM)
C/Lanuza, 19-Lo cal Izquierdo. 28028 MA DRID, SPAIN
Phone +34 91 7130147 - Mo bile +34 690 202 696
Tele fax +34 91 7258059
Email: nachoserrano@celiacosmadrid.org
Dr. Ylva Sjögren Bolin
National Food Ad minis tration Research & Develop ment
P.O. Box 622. SE-751 26 UPPSALA, SWE DEN
Phone +46 18 171416 - Tele fax +46 18 105848
Email: ylva.sjogren@slv.se
Catherine N. Torgler, PhD
Biomedal S.L. Avda. Américo Vespucio 5-E. 1a M-12.
41092 SEVILLA, SPAIN
Phone +34-954 08 12 76 - Tele fax +34-954 08 12 79
Email: catherine.torgler@biomedal.com
Matilde Torralba
Celíacs de Catalunya
Balmes 109, Pral 2a. 08008 BAR CE LONA, SPAIN
Phone +34 93 412 1789 - Tele fax +34 93 487 58 58
Email: mtorralba@celiacscatalunya.org
INVITED SPEAKERS
Prof. Alessio Fasano, M.D.
Professor of Pediatrics, Medicine and Phys i ol ogy
Di rec tor Mucosal Bi ol ogy Re search Cen ter University of Maryland
School of Medicine Health Science Facility II Room S345
20 Penn St. BALTIMORE, MD 21201
Phone +410 706 5501 - Telefax +410 706 5508
E-mail: afasano@mbrc.umaryland.edu
Prof. Dr. Marco Gobbetti
Dipartimento di Biologia e Chimica
Agro-Forestale ed Ambientale Università degli Studi di Bari
Via Amendola 165/A. 70125 BARI, ITALY
Phone +39 070 15 44 29 49
Email: gobbetti@agr.uniba.it
Prof. Dr. Mauro Rossi
Istituto di Scienze dell'Alimentazione
Consiglio Nazionale delle Ricerche
Via Roma 64. 83100 AVELLINO, ITALY
Phone +39 0825 299 391 - Telefax +39 0825 299 104
Email: mrossi@isa.cnr.it
Dr. Umberto Volta
Department of Gastrenterology and Internal Medicine Policlinico S. Orsola-Malpighi
via Massarenti 9. 40 138 BOLOGNA, ITALY
Phone +39 051 6 36 36 33 - Telefax +39 051 34 08 77
Email: umberto.volta@aosp.bo.it
Hertha Deutsch
AOECS. As so ci a tion of Eu ro pean Coeliac So ci et ies - Co dex and Reg u la tory Af fairs
Anton Baumgartner-Straße 44/C5/2302. 1230 VI ENNA, AUS TRIA
Phone +43 1 66 71887 - Tele fax +43 1 66 71887
Email: hertha.deutsch@utanet.at
Rich ard J. Fielder, MBA; CSci FIFST
ROMER LABS UK Lim ited
Building 5325, North Wales Busi ness Park
ABERGELE LL22 8LJ. UK/England
Phone +44 1745 826016 - Tele fax +44 1745 827150
Email: rich ard.fielder@romerlabs.com
Dr. Chris tian Gößwein
R-Biopharm AG. An der neuen Bergstraße 17
64297 DARMSTADT, GER MANY
Phone +49 6151 81 02578 - Tele fax +49 6151 81 02 20
Email: c.goesswein@r-biopharm.de
Marta Gomez Pes
Celíacs de Catalunya
Balmes 109, Pral 2a. 08008 BAR CE LONA, SPAIN
Phone +34 93 412 1789 - Tele fax +34 93 487 58 58
Email: marta@celiacscatalunya.org
Gertrud Granel
Fachverband der Stärke-Industrie e.V.
Königstraße 57. 53115 BONN, GER MANY
Phone +49 30 8871 3398-15 - Tele fax +49 30 8871 3398-19
Email: g.granel@verbaende-jess.de
Tunde Koltai
As so ci a tion of Eu ro pean Coeliac Societies
Rue de la Puere 4. BRUXELLES 1000, BEL GIUM
Phone +36 30 9529965 - Tele fax +36 14380233
Email: theboard@aoecs.org
Email: tuned.koltai@t-on line.de
Arrate Lasa
Uni ver sity of the Basque Coun try. Dept. Nu tri tion & Food Sci ences
Paseo de la Universidad, 7. 01006 VITORIA-GASTEIZ, SPAIN
Phone +34 94 501 3863
Email: arratelasa@gmail.com
Stella Lindeke
R-Biopharm AG An der neuen Bergstraße 17
64297 DARMSTADT, GER MANY
Phone +49 6151 8102 92 - Tele fax +49 6151 8102 40
Email: s.lindeke@r-biopharm.de
An to nio Lo Conte
Istituto di Scienze dell'Alimentazione
Consiglio Nazionale delle Ricerche
Via Roma 64. 83100 AVELLINO, IT ALY
Phone +39 0825 299 391 - Tele fax +39 0825 299 104
Email: aloconte@isa.cnr.it
Giovanni Maria Maggiore
Biodiagene Mar ket ing Man ager BioDiagene
Via Aquilleia, 34A. 90144 PALERMO, IT ALY
Phone +39 91 6932138 - Mo bile +39 334 6867810
Email: gm.mattiore@biodiagene.com
Jac que line Pante
Dr. Schär GmbH/Srl. Winkelau 9
39014 BURGSTALL/POSTAL, IT ALY
Phone +39 0473 293351
Mo bile +39 334 601 3813 - Tele fax +39 0473 293380
Email: jacqueline.pante@schaer.com
MSc. Lea Pollak, Nutr. Tech.
Hrvatski zavod za javno zdravstvo Cro atian National
In stitute of Pub lic Health
Rockefellerova 7. 10000 ZAGREB, KROATIEN
Phone +385 1 4863-266 - Tele fax +385 1 4683-907
Email: lea.pollak@hzjz.hr
Adrian Rog ers
ROMER LABS UK Lim ited. Build ing 5325,
North Wales Busi ness Park. ABERGELE LL22 8LJ. UK/Eng land
Phone +44 (0)845 519 50 10
Email: adrian.rog ers@romerlabs.com
Chiara Scaglione
Progetto Spiga Barrata Italian Coeliac Association
Via Caffaro 68 A rosso. 16124 GENOVA, IT ALY
Phone +39 010 2510235 - Tele fax +39 010 2721615
Email: spigabarrata@celiachia.it
Maren Wiese
Hermann Kröner GmbH. Postfach 1354
49463 IBBENBÜREN/WESTFALEN, GER MANY
Phone +49 5451 9447 12 - Tele fax +49 5451 9447 39
Email: wiese@kroener-staerke.de
Impressum
Pro ceed ings of the 24th Meet ing
WORKING GROUP on PROLAMINANALYSIS and TOXIC ITY
30 Sep tem ber - 2 October, 2010
Ancona, Italy
Wich tiger Hin weis:
Das Werk, einschließlich aller seiner Teile, ist urheberrechtlich geschützt.
Jede Verwertung außerhalb der engen Grenzen des Urheberrechtgesetzes ist ohne Zustimmung des Verlages un zulässig und strafbar. Das gilt ins besondere für Vervielfältigungen, Übersetzungen, Mikroverfilmungen und die Einspeicherung und Verarbeitung in elektronischen Systemen.
Scientific Organisation
Prof. Dr. Martin Stern
Universitätsklinik für Kinder- und Jugendmedizin
Hoppe-Seyler-Straße 1, 72076 TÜBINGEN, GER MANY
Phone +49 7071 2983781; Tele fax +49 7071 295477
Email: martin.stern@med.uni-tuebingen.de
Host
Prof. Dr. Carlo Catassi
Department of Pediatrics
Università Politecnica delle Marche
Co-Director, Center for Celiac Research,
University of Maryland School of Medicine, BALTIMORE MD, USA
Phone +39 071 596 23 64, Mobile +39 349 22 35 447, Telefax +39 071 36281
Email: catassi@tin.it
AiC - Associazione Italiana Celiachia
Via Caffaro 68 A/R, 16124 GENOVA, ITALY
Phone +39 010 25 10 235, Telefax +39 010 27 21 615, www.celiachia.it
Cover picture
Thomas Mothes
2011 Verlag Wissenschaftliche Scripten
www.verlag-wiss-scripten.de
ISBN: 978-3-942267-18-2
|


